Abstract
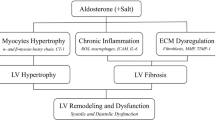
Changes in the composition of cardiac tissue develop in arterial hypertension and lead to structural remodeling of the myocardium. Structural remodeling is the consequence of a number of pathologic processes, mediated by mechanical, neurohormonal and cytokine routes, occurring in the cardiomyocyte and the noncardiomyocyte compartments of the heart. One of these processes is related to the disruption of the equilibrium between the synthesis and degradation of collagen type I and III molecules, which results in an excessive accumulation of collagen type I and III fibers in the interstitium and the perivascular regions of the myocardium. The clinical relevance of ventricular fibrosis is that it might contribute to the increased cardiac risk of patients with hypertensive heart disease. This review focuses on the mechanisms of hypertensive ventricular fibrosis and its clinical consequences. In addition, we discuss the noninvasive methods for the diagnosis of cardiac fibrosis and the therapeutic strategies aimed to promote its reduction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Tanaka M et al. (1986) Quantitative analysis of myocardial fibrosis in normals, hypertensive hearts, and hypertrophic cardiomyopathy. Br Heart J 55: 575–581
Olivetti G et al. (1994) Myocyte cellular hypertrophy is responsible for ventricular remodeling in the hypertrophied heart of middle aged individuals in the absence of cardiac failure. Cardiovasc Res 28: 1199–1208
Rossi MA (1998) Pathologic fibrosis and connective tissue matrix in left ventricular hypertrophy due to chronic arterial hypertension in humans. J Hypertens 16: 1031–1041
Ciulla M et al. (1997) Echocardiographic patterns of myocardial fibrosis in hypertensive patients: Endomyocardial biopsy versus ultrasonic tissue characterization. J Am Soc Echocardiogr 10: 657–664
Schwartzkopff B et al. (2000) Repair of coronary arterioles after treatment with perindopril in hypertensive heart disease. Hypertension 36: 220–225
Brilla CG et al. (2000) Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation 102: 1388–1393
Querejeta R et al. (2000) Serum carboxy-terminal propeptide of procollagen type I is a marker of myocardial fibrosis in hypertensive heart disease. Circulation 101: 1729–1735
Weber KT (2000) Fibrosis and hypertensive heart disease. Curr Opin Cardiol 15: 264–272
Varo N et al. (1999) Losartan inhibits the post-transcriptional synthesis of collagen type I and reverses left ventricular fibrosis in spontaneously hypertensive rats. J Hypertens 17: 107–114
Varo N et al. (2000) Chronic AT1 blockade stimulates extracellular collagen type I degradation and reverses myocardial fibrosis in spontaneously hypertensive rats. Hypertension 35: 1197–1202
Bishop JE and Lindahl G (1999) Regulation of cardiovascular collagen synthesis by mechanical load. Cardiovasc Res 42: 27–44
López B et al. (2004) Role of matrix metalloproteinases in hypertension-associated cardiac fibrosis. Curr Opin Nephrol Hypertens 13: 197–204
Wakatsuki T et al. (2004) The biochemical reponse of the heart to hypertension and exercise. Trends Biochem Sci 29: 609–617
Amanuma S et al. (1994) Biventricular endomyocardial biopsy findings in essential hypertension of graded severity. Postgrad Med J 70 (Suppl 1): S67–S71
Pearlman ES et al. (1982) Muscle fiber orientation and connective tissue content in the hypertrophied human heart. Lab Invest 46: 158–164
Boldt A et al. (2004) Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart 90: 400–405
López B et al. (2001) Usefulness of serum carboxy-terminal propeptide of procollagen type I in assessment of the cardioreparative ability of antihypertensive treatment in hypertensive patients. Circulation 104: 286–291
Díez J et al. (2002) Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation 105: 2512–2517
González A et al. (2002) Regulation of myocardial fibrillar collagen by angiotensin II. A role in hypertensive heart disease? J Mol Cell Cardiol 34: 1585–1593
Díez J (2004) Profibrotic effects of angiotensin II in the heart: a matter of mediators. Hypertension 43: 1164–1165
Tokuda K et al. (2004) Pressure-independent effects of angiotensin II on hypertensive myocardial fibrosis. Hypertension 43: 499–503
Yoshida J et al. (2004) AT1 receptor blocker added to ACE inhibitor provides benefits at advanced stage of hypertensive diastolic failure. Hypertension 43: 686–691
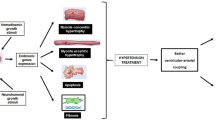
Lijnen P and Petrov V (2000) Induction of cardiac fibrosis by aldosterone. J Mol Cell Cardiol 32: 865–879
Brilla CG et al. (1993) Antifibrotic effect of spironolactone in preventing myocardial fibrosis in systemic arterial hypertension. Am J Cardiol 71: 12A–16A
Brilla CG et al. (1994) Collagen metabolism in cultured adult rat cardiac fibroblasts: response to angiotensin II and aldosterone. J Mol Cell Cardiol 26: 809–820
Rocha R et al. (2002) Selective aldosterone blockade prevents angiotensin II/salt-induced vascular inflammation in the rat heart. Endocrinology 143: 4828–4836
Iglarz M et al. (2004) Involvement of oxidative stress in the profibrotic action of aldosterone. Interaction with the renin-angiotensin system. Am J Hypertens 17: 597–603
Kuster GM et al. (2005) Mineralocorticoid receptor inhibition ameliorates the transition to myocardial failure and decreases oxidative stress and inflammation in mice with chronic pressure overload. Circulation 111: 420–427
Ahokas RA et al. (2003) Aldosteronism and peripheral blood mononuclear cell activation: a neuroendocrine-immune interface. Circ Res 93: e124–e135
Jacob HJ et al. (1991) Genetic mapping of a gene causing hypertension in the stroke-prone spontaneously hypertensive rat. Cell 67: 213–224
Challah M et al. (1998) Angiotensin I-converting enzyme genotype influences arterial response to injury in normotensive rats. Arterioscler Thromb Vasc Biol 18: 235–243
Ocaranza MP et al. (2004) Polymorphism in gene coding for ACE determines different development of myocardial fibrosis in rats. Am J Physiol Heart Circ Physiol 286: H498–H506
Díez J et al. (2003) The A1166C polymorphism of the AT1 receptor gene is associated with collagen type I synthesis and myocardial stiffness in hypertensives. J Hypertens 21: 2085–2092
Frohlich ED (2001) Fibrosis and ischemia: the real risks in hypertensive heart disease. Am J Hypertens 14: 194S–199S
Burlew BS and Weber KT (2002) Cardiac fibrosis as a cause of diastolic dysfunction. Herz 27: 92–98
Burlew BS and Weber KT (2000) Connective tissue and the heart. Functional significance and regulatory mechanisms. Cardiol Clin 18: 435–442
Ohsato K et al. (1992) Histopathologic factors related to diastolic function in myocardial hypertrophy. Jpn Circ J 56: 325–333
McLenachan JM and Dargie JH (1990) Ventricular arrhythmias in hypertensive left ventricular hypertrophy. Relation to coronary artery disease, left ventricular dysfunction, and myocardial fibrosis. Am J Hypertens 3: 735–740
Querejeta R et al. (2004) Increased collagen type I synthesis in patients with heart failure of hypertensive origin: relation to myocardial fibrosis. Circulation 110: 1263–1268
Schwartzkopff B et al. (1993) Structural and functional alterations of the intramyocardial coronary arterioles in patients with arterial hypertension. Circulation 88: 993–1003
Isoyama S et al. (1992) Collagen deposition and the reversal of coronary reserve in cardiac hypertrophy. Hypertension 20: 491–500
Bethge C et al. (1987) Ventricular arrhythmias in hypertensive heart disease with and without heart failure. J Cardiovasc Pharmacol 10 (Suppl 6): S119–S128
Nattel S (2004) Defining “culprit mechanisms” in arrhythmogenic cardiac remodeling. Circ Res 94: 1403–1405
Kannel WB et al. (1982) Epidemiologic features of chronic atrial fibrillation. The Framingham Study. N Engl J Med 306: 1018–1022
Tamirisa PK et al. (2001) Ultrasonic tissue characterization: review of an approach to assess hypertrophic myocardium. Echocardiography 18: 593–597
Kerut EK et al. (2003) Review of methods for texture analysis of myocardium from echocardiographic images: a means of tissue characterization. Echocardiography 20: 727–736
Picano E et al. (1990) In vivo quantitative ultrasonic evaluation of myocardial fibrosis in humans. Circulation 81: 58–64
Moon JC and Prasad SK (2005) Cardiovascular magnetic resonance and the evaluation of heart failure. Curr Cardiol Rep 7: 39–44
Tandri H et al. (2005) Noninvasive detection of myocardial fibrosis in arrhythmogenic right ventricular cardiomyopathy using delayed-enhancement magnetic resonance imaging. J Am Coll Cardiol 45: 98–103
López B et al. (2001) Biochemical assessment of myocardial fibrosis in hypertensive heart disease. Hypertension 38: 1222–1226
Díez J et al. (1996) Serum markers of collagen type I metabolism in spontaneously hypertensive rats: relation to myocardial fibrosis. Circulation 93: 1026–1032
Guidelines Committee (2003) European Society of Hypertension–European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens 21: 1011–1053
Brilla CG et al. (1991) Cardioreparative effects of lisinopril in rats with genetic hypertension and left ventricular hypertrophy. Circulation 83: 1771–1779
Kagitani S et al. (2004) Tranilast attenuates myocardial fibrosis in association with suppression of monocyte/macrophage infiltration in DOCA/salt hypertensive rats. J Hypertens 22: 1007–1015
Rasoul S et al. (2004) Antifibrotic effect of Ac-SDKP and angiotensin-converting enzyme inhibition in hypertension. J Hypertens 22: 593–603
Ogata T et al. (2004) Myocardial fibrosis and diastolic dysfunction in deoxycorticosterone acetate-salt hypertensive rats is ameliorated by the peroxisome proliferators-activated receptor-alpha activator fenofibrate, partly by suppressing inflammatory responses associated with the nuclear factor-kappa-B pathway. J Am Coll Cardiol 43: 1481–1488
Meiners S et al. (2004) Downregulation of matrix metalloproteinases and collagens and suppression of cardiac fibrosis by inhibition of the proteasome. Hypertension 44: 471–47755
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- EXTRACELLULAR MATRIX
-
A complex network of polysaccharides and proteins secreted by cells that serves as a structural element in tissues and influences cell physiology
- COLLAGEN TYPE I
-
A major component of the extracellular matrix, rich in glycine and proline; the most abundant collagen type present in the heart
- PROTEIN KINASE B
-
A member of the protein kinase family, that transfers one terminal phosphate group of ATP to a specific amino acid of a target protein
- EXTRACELLULAR-SIGNAL-REGULATED KINASES
-
Serine or threonine kinases activated by various extracellular signals that induce proliferation and differentiation; also called mitogen-activated kinases
- TISSUE-INTEGRATED BACKSCATTER
-
Measurement of changes in ultrasonic attenuation with an index of backscatter to distinguish normal from abnormal (i.e. fibrotic) myocardium
- PROCOLLAGEN TYPE I
-
Molecule secreted by fibroblasts into the extracellular space, where it is converted to collagen type I by specific endoproteinases
- UBIQUITIN-PROTEASOME SYSTEM
-
Type of large-protein complex in the cytosol that is responsible for degrading proteins that have been marked for destruction by ubiquitination
Rights and permissions
About this article
Cite this article
Díez, J., González, A., López, B. et al. Mechanisms of Disease: pathologic structural remodeling is more than adaptive hypertrophy in hypertensive heart disease. Nat Rev Cardiol 2, 209–216 (2005). https://doi.org/10.1038/ncpcardio0158
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpcardio0158
This article is cited by
-
Role of cardiovascular magnetic resonance in the clinical evaluation of left ventricular hypertrophy: a 360° panorama
The International Journal of Cardiovascular Imaging (2022)
-
Human pluripotent stem cell–based cardiovascular disease modeling and drug discovery
Pflügers Archiv - European Journal of Physiology (2021)
-
Relationships between kidney dysfunction and left ventricular diastolic dysfunction: a hospital-based retrospective study
Journal of Nephrology (2021)
-
Sensitive marker for evaluation of hypertensive heart disease: extracellular volume and myocardial strain
BMC Cardiovascular Disorders (2020)
-
Sporadic inclusion body myositis: no specific cardiac involvement in cardiac magnetic resonance tomography
Journal of Neurology (2020)