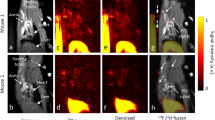
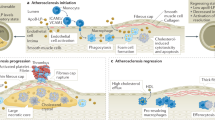
Abstract
Identification of high-risk atherosclerotic lesions prone to rupture and thrombosis may greatly decrease the morbidity and mortality associated with atherosclerosis. The development of magnetic resonance imaging contrast agents that specifically target components of the atherosclerotic plaque might enable non-invasive detection of high-risk lesions. This review discusses a variety of molecules present in atherosclerotic plaque that could serve as targets for specific contrast agents. Ultimately, such agents may allow the identification of high-risk atherosclerotic lesions in patients and enable treatment of these patients before lesion progression and complications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
American Heart Association (2004) Heart Disease and Stroke Statistics—2004 Update. [http://www.americanheart.org/downloadable/heart /1079736729696HDSStats2004UpdateREV3-19-04.pdf] (accessed 11 Aug 04)
Malek AM et al. (1999) Hemodynamic shear stress and its role in atherosclerosis. JAMA 282: 2035–2042
Vanhoutte PM (2003) Endothelial dysfunction and atherosclerosis. Eur Heart J 18: E19–29
Cushing S et al. (1990) Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. PNAS 87: 5134–5138
Goldstein JL et al. (1979) Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci USA 76: 333–337
Ross R (1993) The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362: 801–809.
Richardson PD et al. (1989) Influence of plaque configuration and stress distribution on fissuring of coronary atherosclerotic plaques. Lancet 2: 941–944
Falk E (1983) Plaque rupture with severe pre-existing stenosis precipitating coronary thrombosis: characteristics of coronary atherosclerotic plaques underlying fatal occlusive thrombi. Br Heart J 50: 127–134
Jones CB et al. (2003) Matrix metalloproteinases: a review of their structure and role in acute coronary syndrome. Cardiovasc Res 59: 812–823
Nunn AD et al. (1997) Can receptors be imaged with MRI agents? Q J Nucl Med 41: 155–162
Aime S et al. (2002) Insights into the use of paramagnetic Gd(III) complexes in MR-molecular imaging investigations. J Magn Reson Imaging 16: 394–406
Suzuki H et al. (1997) A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature 386: 292–296
Gough PJ et al. (1999) Analysis of macrophage scavenger receptor (SR-A) expression in human aortic atherosclerotic lesions. Arterioscler Thromb Vasc Biol 19: 461–471
Nakata A et al. (1999) CD36, a novel receptor for oxidized low-density lipoproteins, is highly expressed on lipid-laden macrophages in human atherosclerotic aorta. Arterioscler Thromb Vasc Biol 19: 1333–1339
Weissleder R et al. (1990) Ultrasmall superparamagnetic iron oxide: characterization of a new class of contrast agents for MR imaging. Radiology 175: 489–493
Saini S et al. (1987) Ferrite particles: a superparamagnetic MR contrast agent for the reticuloendothelial system. Radiology 162: 211–216
Weissleder R et al. (1990) Ultrasmall superparamagnetic iron oxide: an intravenous contrast agent for assessing lymph nodes with MR imaging. Radiology 175: 494–498
Litovsky S et al. (2003) Superparamagnetic iron oxide-based method for quantifying recruitment of monocytes to mouse atherosclerotic lesions in vivo: enhancement by tissue necrosis factor-α, interleukin-1β, and interferon-γ. Circulation 107: 1545–1549
Kanno S et al. (2001) Macrophage accumulation associated with rat cardiac allograft rejection detected by magnetic resonance imaging with ultrasmall superparamagnetic iron oxide particles. Circulation 104: 934–938
Ruehm SG et al. (2001) Magnetic resonance imaging of atherosclerotic plaque with ultrasmall superparamagnetic particles of iron oxide in hyperlipidemic rabbits. Circulation 103: 415–422
Kooi ME et al. (2003) Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation 107: 2453–2458
Tsimikas S (2002) Noninvasive imaging of oxidized low-density lipoprotein in atherosclerotic plaques with tagged oxidation-specific antibodies. Am J Cardiol 90: 22L–27L
Ehara S et al. (2001) Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation 103: 1955–1960
Tsimikas S et al. (1999) Radiolabeled MDA2, an oxidation-specific, monoclonal antibody, identifies native atherosclerotic lesions in vivo. J Nucl Cardiol 6: 41–53
Tsimikas S et al. (2000) In vivo uptake of radiolabeled MDA2, an oxidation-specific monoclonal antibody, provides an accurate measure of atherosclerotic lesions rich in oxidized LDL and is highly sensitive to their regression. Arterioscler Thromb Vasc Biol 20: 689–697
Brooks PC et al. (1994) Requirement of vascular Radiology 221: 237–243
O'Brien ER et al. (1994) Angiogenesis in human coronary atherosclerotic plaques. Am J Pathol 145: 883–894
Winter PM et al. (2003) Molecular imaging of angiogenesis in early-stage atherosclerosis with αvβ3-integrin-targeted nanoparticles. Circulation 108: 2270–2274
Winter PM et al. (2003) Molecular imaging of angiogenesis in nascent Vx-2 rabbit tumors using a novel αvβ3-targeted nanoparticle and 1.5 tesla magnetic resonance imaging. Cancer Res 63: 5838–5843
Anderson SA et al. (2000) Magnetic resonance contrast enhancement of neovasculature with αvβ3-targeted nanoparticles. Magn Reson Med 44: 433–439
Volker W et al. (1989) Cytochemical changes in a human arterial proteoglycan related to atherosclerosis. Atherosclerosis 77: 117–130
Kolodgie FD et al. (2002) Differential accumulation of proteoglycans and hyaluronan in culprit lesions: insights into plaque erosion. Arterioscler Thromb Vasc Biol 22: 1642–1648
Wallner K et al. (1999) Tenascin-C is expressed in macrophage-rich human coronary atherosclerotic plaque. Circulation 99: 1284–1289
Aguinaldo JGS et al. (2002) Atheroslcerotic plaque imaging using a novel contrast agent gadofluorine M [Abstract]. Mol Imaging 2: s282
Barkhausen J et al. (2003) Detection of atherosclerotic plaque with gadofluorine-enhanced magnetic resonance imaging. Circulation 108: 605–609
Sirol M et al. (2004) Lipid-rich atherosclerotic plaques detected by gadofluorine-enhanced in vivo magnetic resonance imaging. Circulation 109: 2890–2896
Brown DL et al. (1995) Identification of 92-kD gelatinase in human coronary atherosclerotic lesions: association of active enzyme synthesis with unstable angina. Circulation 91: 2125–2131
Cipollone F et al. (2001) Overexpression of functionally coupled cyclooxygenase-2 and prostaglandin E synthase in symptomatic atherosclerotic plaques as a basis of prostaglandin E(2)-dependent plaque instability. Circulation 104: 921–927
Diamant M et al. (2004) Cellular microparticles: new players in the field of vascular disease? Eur J Clin Invest 34: 392–401
Jonasson L et al. (1986) Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis 6: 131–138
Mallat Z et al. (1999) Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: a role for apoptosis in plaque thrombogenicity. Circulation 99: 348–353
Himber J et al. (2003) Inhibition of tissue factor limits the growth of venous thrombus in the rabbit. J Thromb Haemost 1: 889–895
Morawski AM et al. (2004) Targeted nanoparticles for quantitative imaging of sparse molecular epitopes with MRI. Magn Reson Med 51: 480–486
Flacke S et al. (2001) Novel MRI contrast agent for molecular imaging of fibrin: implications for detecting vulnerable plaques. Circulation 104: 1280–1285
Winter PM et al. (2003) Improved molecular imaging contrast agent for detection of human thrombus. Magn Reson Med 50: 411–416
Yu X et al. (2000) High-resolution MRI characterization of human thrombus using a novel fibrin-targeted paramagnetic nanoparticle contrast agent. Magn Reson Med 44: 867–872
Schmitz SA et al. (2001) USPIO-enhanced direct MR imaging of thrombus: preclinical evaluation in rabbits. Radiology 221: 237–243
Johansson LO et al. (2001) A targeted contrast agent for magnetic resonance imaging of thrombus: implications of spatial resolution. J Magn Reson Imaging 13: 615–618
Jaffer FA and Weissleder R (2004) Seeing within: molecular imaging of the cardiovascular system. Circ Res 94: 433–445
Fayad ZA et al. (2002) Detection of arterial thrombi in vivo by MRI using a fibrin-targeted contrast agent [Abstract II–435]. Circulation 106
Acknowledgements
Marc Sirol helped with this paper and MR images. MJL received funding from Stanley J Sarnoff Endowment, and ZAF from NIH/NHLBI R01 HL71021 and HL078667.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lipinski, M., Fuster, V., Fisher, E. et al. Technology Insight: targeting of biological molecules for evaluation of high-risk atherosclerotic plaques with magnetic resonance imaging. Nat Rev Cardiol 1, 48–55 (2004). https://doi.org/10.1038/ncpcardio0013
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpcardio0013
This article is cited by
-
Imaging Atherosclerotic Plaques with MRI: Role of Contrast Agents
Current Cardiovascular Imaging Reports (2013)
-
Molecular imaging in cardiovascular disease: targets and opportunities
Nature Reviews Cardiology (2009)
-
Identification of interleukin-2 for imaging atherosclerotic inflammation
European Journal of Nuclear Medicine and Molecular Imaging (2006)