Abstract
Two-dimensional polymeric nanosheets have recently gained much attention, particularly top-down nanosheets such as graphene and metal chalcogenides originating from bulk-layered mother materials. Although molecule-based bottom-up nanosheets manufactured directly from molecular components can exhibit greater structural diversity than top-down nanosheets, the bottom-up nanosheets reported thus far lack useful functionalities. Here we show the design and synthesis of a bottom-up nanosheet featuring a photoactive bis(dipyrrinato)zinc(II) complex motif. A liquid/liquid interfacial synthesis between a three-way dipyrrin ligand and zinc(II) ions results in a multi-layer nanosheet, whereas an air/liquid interfacial reaction produces a single-layer or few-layer nanosheet with domain sizes of >10 μm on one side. The bis(dipyrrinato)zinc(II) metal complex nanosheet is easy to deposit on various substrates using the Langmuir–Schäfer process. The nanosheet deposited on a transparent SnO2 electrode functions as a photoanode in a photoelectric conversion system, and is thus the first photofunctional bottom-up nanosheet.
Similar content being viewed by others
Introduction
Nanosheets with two-dimensional polymeric structures have recently attracted significant attention. Graphene, a prominent nanosheet with various attractive properties, has been investigated extensively for applications in electronics1,2,3, photonics4 and spintronics5,6. The broad attention and expected utility of graphene have stimulated research into other nanosheets, such as metal oxides7,8,9,10,11, metal sulfides12,13,14,15 and metal hydroxides16,17,18,19,20, which also exhibit desirable and useful properties (for example, semiconductivity10,15 and ferroelectricity8,14). These nanosheets originate from bulk-layered crystalline mother materials (that is, top-down nanosheets).
Another type of nanosheets, namely molecule-based bottom-up nanosheets, is emerging. This series of nanosheets is fabricated directly from atomic, ionic and molecular components. The concept was proposed nearly a century ago, although it has only recently been realized21. For example, Schlüter and King created independently single-layer cycloaddition-induced anthracene22,23,24,25 nanosheets that featured carbon–carbon covalent bonds. 2,2′:6′,2′′-Terpyridine/metal complex nanosheets based on a metal–ligand coordination linkage were reported by Schlüter26,27. Frauenrath synthesized a carbon-rich nanosheet using carbonization of an amphiphilic hexayne molecule28. Other examples include surface metal-organic frameworks29,30, surface covalent-organic frameworks31,32,33,34, metal-surface-mediated monolayer formation in the vacuum phase35,36,37,38,39, and single-layer or few-layer metal-organic frameworks and covalent-organic frameworks delaminated from the crystal phase40,41,42,43,44,45,46. A significant advantage of the bottom-up synthesis is that structures can be customized through the selection of components (for example, metal ions and organic ligand molecules). Therefore, the bottom-up approach may broaden the diversity and utility of nanosheets. Although previous reports on bottom-up nanosheets have concentrated on the fabrication and analysis of various two-dimensional structures, no functionality has yet been demonstrated.
Given this background, we have sought to create functional bottom-up nanosheets, including an electrically conductive nickel bis(dithiolene) nanosheet47,48,49. The present work describes a bis(dipyrrinato)zinc(II) complex nanosheet synthesized from a three-way dipyrrin ligand and zinc(II) acetate. The spontaneous and reversible coordination of dipyrrin ligands with metal ions50,51 makes them suitable as building blocks for bottom-up nanosheets. The bis(dipyrrinato)zinc(II) complex motif acts not only as a connecting point but also as a photofunctional moiety: it has a strong absorption in the visible and near-infrared region (tunable by substituents)52,53,54. In addition to the synthesis and identification of the bis(dipyrrinato)zinc(II) complex nanosheet, we report its large domain size (sides of >10 μm), guest inclusion, stepwise layering and photoelectric conversion ability.
Results
Synthesis and morphology of multi-layer N1
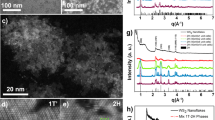
Figure 1 depicts bis(dipyrrinato)zinc(II) complex nanosheet N1 synthesized from three-way dipyrrin ligand L1 and zinc(II) acetate. To verify the macroscopic formation of N1, the first process considered was a liquid/liquid interfacial synthesis47 using an aqueous zinc(II) acetate solution (upper layer, 5.0 × 10−2 mol l−1) and a dichloromethane solution of L1 (lower layer, 1.0 × 10−4 mol l−1; Fig. 2a). A spontaneous reaction at room temperature led to the generation of multi-layer N1 at the water/oil interface, which appeared as an orange film. Multi-layer N1 may be transferred from the interface onto various substrates. For example, on an indium tin oxide (ITO) substrate it appears as a transparent film (Fig. 2a). Multi-layer N1 is not soluble in either water or organic solvent, reflecting the polymeric structure proposed in Fig. 1. Optical and scanning electron microscopic images reveal a uniform, flat, film-like morphology (Fig. 2b,c). The presence of cracks and wrinkles also indicates the sheet structure. The typical thickness of multi-layer N1 on a silicon(111) substrate modified with 1,1,1,3,3,3-hexamethyldisilazane (HMDS/Si(111)) was observed to be 700 nm by atomic force microscopy (AFM, Fig. 2d), which corresponds to ~580 layers considering the thickness of single-layer N1 (vide infra). The thickness of N1 may be controlled by the concentration of L1 in dichloromethane and may span 6–800 nm (5–670 layers) using L1 solutions with concentrations of 1.0 × 10−6 to 5.0 × 10−4 mol l−1 (Fig. 2e). We note that the liquid/liquid interfacial synthesis is essential for the synthesis of multi-layer N1. A conventional single-phase synthesis, performed in either dichloromethane at room temperature or N,N-dimethylformamide at 105 °C, resulted in a solid material far from a film texture (Fig. 2f,g). In fact, its disordered structure was verified by X-ray photoelectron spectroscopy (XPS, vide infra).
(a) Schematic illustration and photographs of the liquid/liquid interfacial synthesis and multi-layer N1 transferred onto an ITO substrate. Scale bars, 5 and 1 mm, respectively. (b) Optical microscopic image on an ITO substrate. Scale bar, 50 μm. (c) Field-emission scanning electron microscopic (FE-SEM) image on HMDS/Si(111). Scale bar, 20 μm. (d) Atomic force microscopic image on HMDS/Si(111) and its cross-section analysis along the magenta line. Scale bar, 5 μm. (e) Control of the thickness based on the concentration of L1 in the liquid/liquid interfacial synthesis. The inset shows a close-up of the low concentration region. Reaction time, 4 days. Temperature, room temperature. Container, cylindrical glass vial with a diameter of 3.2 cm. Volume of the upper (aqueous) layer, 20 ml. Volume of the lower (dichloromethane) layer, 10 ml. Concentration of zinc(II) acetate in the upper layer, 0.05 mol l−1. (f,g) Photographs of the products of single-phase reactions in dichloromethane at room temperature and in N,N-dimethylformamide at 105 °C, respectively.
Synthesis of single-layer or few-layer N1
Next, we synthesized single-layer N1. Figure 3a shows the fabrication procedure, an air/liquid interfacial synthesis47,48,49. A very tiny amount (5 μl) of a dilute dichloromethane solution of L1 (7.4 × 10−5 mol l−1) was gently dropped onto the surface of an aqueous phase containing zinc(II) acetate (5.0 × 10−2 mol l−1) at ambient temperature. After prompt evaporation of dichloromethane, spontaneous air/water interfacial complexation occurred between L1 and zinc(II) ions, and single-layer N1 was produced at the interface. The resultant single-layer N1 was almost invisible to the naked eye but could be transferred onto various substrates via horizontal deposition known as the Langmuir–Schäfer method (Fig. 3). We note that few-layer N1 was also synthesized by increasing the dichloromethane solution of L1 to 20 μl. The single-layer or few-layer N1 on a flat substrate was then subjected to a series of analyses.
XPS of N1
XPS was conducted for single-layer N1 on HMDS/Si(111) to assess its constituent elements (N and Zn) and their bonding properties (Fig. 4a,b). For reference, dipyrrin ligand L1, mononuclear bis(dipyrrinato)zinc(II) complex M1 (Fig. 4c) and multi-layer N1 deposited on highly ordered pyrolitic graphite (HOPG) were also subjected to XPS. Ligand L1 did not show a Zn 2p3/2 peak, whereas the peak was present at similar binding energies in the spectra of M1, multi-layer N1 and single-layer N1 (1021.8, 1021.6 and 1021.8 eV, respectively). The spectrum of L1 features two N 1s peaks (400.0 and 398.4 eV), assignable to pyrrolic and iminic nitrogen atoms, respectively55, whereas M1 shows a single N 1s peak at 398.8 eV, which originates from its coordination to the zinc(II) centre, which makes the two nitrogen atoms equivalent to one another. Both multi-layer N1 and single-layer N1 displayed a single peak for N 1s at almost the same binding energy as M1 (399.0 eV). In addition, the abundance ratio calculated from the peak area corrected using the photoionization cross-section is consistent with the ideal value of N:Zn=4:1 (80.6:19.4, 80.7:19.3 and 79.1:20.9 for M1, multi-layer N1 and single-layer N1, respectively). These findings indicate that the bis(dipyrrinato)zinc(II) complex motif formed quantitatively in the nanosheet. We note that coordination of H2O molecule(s) to the zinc(II) centre to form a square–pyramidal or trans-octahedral coordination sphere may be excluded by the fact that multi-layer N1 on oxygen-free HOPG lacked an O 1s peak in XPS (Supplementary Fig. 1). Also noteworthy is that the products of the single-phase reactions (Fig. 2f,g) exhibited nitrogen abundancies in excess of the ideal stoichiometric ratio (N:Zn=5.4:1 and 5.8:1, Supplementary Fig. 2). This result indicates that the single-phase reaction products leave uncoordinating dipyrrin moieties, thereby possessing disordered structures.
(a) Narrow-scan XPS focusing on the N 1s region. (b) Focusing on the Zn 2p3/2 region. Legend: L1 on HMDS/Si(111) (black), M1 on adhesive carbon tape (turquoise), multi-layer N1 on HOPG (blue) and single-layer N1 on HMDS/Si(111) (magenta). The intensity is proportional to the element abundance: the original signal is standardized using the photoionization cross-section of each element. (c) Mononuclear bis(dipyrrinato)zinc(II) reference complex M1.
AFM of single-layer or few-layer N1
The AFM analysis revealed the flat sheet texture and morphology of single-layer N1. Figure 5a,b shows the height and phase images, respectively, of single-layer N1 on an HMDS/Si(111) substrate. The phase image clearly distinguishes single-layer N1 and the bare substrate: the nanosheet possesses a phase value that is lower by 2.4°. The height image shows a domain with one side >10-μm long, which is a noteworthy size for bottom-up nanosheets29,30,31,32,33,34,35,36,37,38,39. To confirm the single-layer nature of the sheet, part of N1 was scratched with the AFM tip at a force of 8.6 × 102 nN. Figure 5c,d shows the height images before and after scratching, and the scratched region is highlighted with a blue square. This treatment resulted in the removal of N1, leaving an intact HMDS/Si(111) surface. A cross-section analysis traversing one of the scratched edges demonstrated that N1 was 1.2-nm thick (Fig. 5e), which is consistent with the size of the bis(dipyrrinato)zinc(II) complex motif. A higher-force scratch (1.2 × 104 nN) resulted in a more drastic change in the AFM image (Supplementary Fig. 3). In this case, both single-layer N1 and the HMDS/Si(111) surface were destroyed.
Few-layer N1 with larger domain sizes was fabricated by increasing the amount of L1 (Supplementary Fig. 4). The phase image is flat, whereas the topological image reveals steps and divided domains. This result indicates that the scanned area was completely covered with few-layer N1. The wrinkles in the layers may be evidence of the sheet structure and were removed by thermal annealing at 120°C (Supplementary Fig. 5).
In-plane periodicity
Scanning tunnelling microscopy (STM) revealed that single-layer N1 on HOPG exhibited a moiré pattern composed of two lattices: one is the in-plane hexagonal periodicity of N1 and the other is derived from HOPG (Supplementary Fig. 6). The in-plane periodicity was also confirmed by selected area electron diffractions (SAEDs) in transmission electron microscopy for multi-layer N1 (Supplementary Fig. 7). They represent two sets of hexagonal diffractions, which are consistent with in-plane diffraction patterns reproduced from crystal lattices comprising piles of single-layer N1, which were optimized using a molecular mechanics calculation (Supplementary Fig. 8; Supplementary Information). This series of analyses ensures the hexagonal in-plane periodicity of N1.
Optical properties and layering
Figure 6a presents the ultraviolet/visible spectra of L1 and M1 in toluene and of few-layer N1 on a quartz substrate. Ligand L1 displayed an intense absorption band at 446 nm, which is derived from the 1π–π* transition of the dipyrrin π-system. Complexation with a zinc(II) ion is known to induce a redshift in the 1π–π* band53, and in fact M1 displayed a 49-nm wavelength shift relative to L1. Few-layer N1 also showed an absorption band in the visible region, with the absorption maximum being closer to that of M1 than to that of L1, which also indicates that the complexation of L1 with zinc(II) ions was complete. Using the 1π–π* band as a probe, single-layer N1 was accumulated stepwise on a quartz substrate. The single-layer nanosheet was fabricated on the air/water interface of a Langmuir–Blodgett trough, which was deposited repeatedly on a quartz substrate at a constant surface pressure using the Langmuir–Schäfer method. Figure 6b presents the ultraviolet/visible spectra of the modified quartz substrate. The peak absorbance of the 1π–π* band at 500 nm is proportional to the number of deposition processes (Fig. 6c), which indicates the quantitative, layer-by-layer accumulation of single-layer N1.
(a) Spectra of L1 (black) and M1 (turquoise) in toluene and few-layer N1 (magenta) on a quartz substrate. (b) Spectral change on stepwise depositions of single-layer N1 on a quartz substrate. (c) Linear relationship between the absorbance at 500 nm and the number of deposition processes. The magenta dots are extracted from Fig. 6b, and the black solid line corresponds to the least-squares linear fit of the plots.
Guest inclusion
Uptake of a fluorescent dye, Rhodamine B, to N1 with large pores was demonstrated (Supplementary Fig. 9). On immersing multi-layer N1 on an ITO or quartz substrate in a dichloromethane solution of Rhodamine B, N1 was stained the colour of the dye (Supplementary Fig. 9a). Guest-incorporated N1 displayed fluorescence from Rhodamine B (Supplementary Fig. 9b,c).
Photoelectric conversion
N1 was employed as the active layer of a photoanode to demonstrate its functionality. A transparent SnO2 working electrode was decorated with 36-layer N1, and a three-electrode system was set up (Supplementary Fig. 10). Triethanolamine (TEOA) was added to an electrolyte solution as a sacrificial electron donor. An anodic current was observed only when the working electrode was irradiated with 500-nm light, corresponding to the absorption maximum of N1 (Fig. 7a). The action spectrum shown in Fig. 7b demonstrates that the photocurrent was maximized with 500-nm light, and no response was observed using light at λ<420 nm or λ>560 nm, a region of negligible absorption for N1. Control experiments lacking either N1 or TEOA did not show current responses at all (Fig. 7c,d). Therefore, the observed photocurrent is derived from the photocatalytic oxidation of TEOA sensitized by N1. Surprisingly, to the best of our knowledge, this report is the first on photoelectric conversion using a bis(dipyrrinato)zinc(II) complex sensitizer despite its excellent light absorption ability. We then studied the relationship between the quantum yield of the photoelectric conversion and thickness of N1 (Fig. 7e; Supplementary Fig. 11). Single-layer N1 exhibited the highest value (0.86%), which decreases gradually with the growth of the thickness of N1, leading to negligible photoresponses at thicknesses of over ~300 layers. For the photocurrent, the maximum is located at ~100–150 layers (Supplementary Fig. 12). To demonstrate the superiority of N1, we also prepared the two types of mononuclear bis(dipyrrinato)zinc(II) complex sensitizers: plain zinc(II) complex M2, which was dropcasted onto a SnO2 electrode to form a physisorbed film (Supplementary Fig. 13a) and M3 with carboxy groups, which underwent chemisorption onto a SnO2 surface to form a self-assembled monolayer (Supplementary Fig. 14a). These two photoanodes resulted in much lower conversion efficiencies (0.030 and 0.069%, Supplementary Figs 13 and 14), thereby justifying the superiority of bottom-up nanosheet N1 over conventional molecular films. The nanosheet structure of N1 affords appropriate porosity and suppresses molecular aggregation; these features presumably make N1 a better sensitizer. In addition, the coexistence of insolubility (to avoid redissolution into media) and manipulability (to facilitate deposition and layering) of N1 is also advantageous for potential applications.
(a) Typical anodic current response on irradiation of a working electrode (SnO2 substrate modified with 36-layer N1) with intermittent 500-nm light. (b) Action spectrum for the photocurrent generation (magenta dots) and absorption spectrum of N1 (black solid line). (c) Control experiment without N1. (d) Control experiment without TEOA. The irradiation pattern is the same as that of a. (e) Relationship between the relative quantum yield (QY) and thickness of N1 on irradiation with 500-nm light. The highest QY (0.86% by single-layer N1) is taken as the standard.
Discussion
A photofunctional bottom-up nanosheet containing the photoactive bis(dipyrrinato)zinc(II) complex motif was fabricated. A nanosheet with atomic thickness was prepared via an air/water interfacial synthesis, during which spontaneous complexation proceeded between a three-way dipyrrin ligand and zinc(II) ions at the interface. The nanosheet was identified using ultraviolet/visible spectroscopy and XPS, which revealed the complete formation of the bis(dipyrrinato)zinc(II) complex motif. The single-layer nanosheet was confirmed by AFM via a scratch experiment. The domain size of the single-layer nanosheet reached 10 μm on one side, which is large for bottom-up nanosheet materials. Repeated deposition of the single-layer nanosheet on a flat substrate resulted in its quantitative layering. The nanosheet efficiently collectes visible light at around 500 nm, and that physisorbed on a transparent SnO2 functioned as an active layer in a photoelectric conversion system. The photo-functionality in the molecule-based bottom-up nanosheet demonstrated herein leads to a significant expansion of the applicability of this type of two-dimensional matters as useful and promising nanomaterials.
Methods
Materials
5′-(4-Formylphenyl)-[1,1′:3′,1′′-terphenyl]-4,4′′-dicarbaldehyde56, 2-methylpyrrole57, 2-((3,5-dimethyl-2H-pyrrol-2-ylidene)(2,6-dimethylphenyl)methyl)-3,5-dimethyl-1H-pyrrole54 and 2-methyl-5-((5-methyl-2H-pyrrol-2-ylidene)(phenyl)methyl)-1H-pyrrole58 were synthesized according to previous reports. Dichloromethane, acetonitrile and ethanol for the interfacial synthesis of N1, pretreatments of substrates and photoelectric conversion were supplied from Kanto Chemical Co., Inc. as high-performance liquid chromatography grade and were used as received. Water was purified using a Milli-Q purification system (Merck KGaA). Tetra-n-butylammonium perchlorate as a supporting electrolyte was purified by recrystallization from ethanol, which was dried in vacuo. Solvents for organic syntheses were purified using a solvent purification system (Ultimate Solvent System, Nikko Hansen & Co., Ltd). The other chemicals were general grades and were used as received. All procedures were conducted under an ambient condition otherwise stated.
Apparatus for the identification of molecular compounds
1H (500 or 400 MHz) and 13C (125 or 100 MHz) nuclear magnetic resonance (NMR) spectra were recorded on a Bruker-DRX500, JEOL ECX-400 or JEOL AL-400 spectrometer. Fast atom bombardment mass spectrometry (FAB-MS) and electrospray ionization time-of-flight spectrometry were conducted using a JEOL JMS-700 MStation and Micromass LCT Premier XE mass spectrometer, respectively.
Synthesis of L1
5′-(4-Formylphenyl)-[1,1′:3′,1′′-terphenyl]-4,4′′-dicarbaldehyde (450 mg, 1.2 mmol) and 2-methylpyrrole (0.63 ml, 7.5 mmol) were dissolved in dichloromethane (100 ml) under a nitrogen atmosphere. One drop of trifluoroacetic acid was added, and the solution changed from light yellow to bright red and was stirred at room temperature for 3 h. When complete consumption of the aldehyde was confirmed by thin-layer chromatography, a solution of chloranil (848 mg, 3.4 mmol) in dichloromethane was added, and the resultant mixture was stirred for an additional 15 min. The reaction mixture was washed with water, dried over magnesium sulfate, filtered and evaporated. The crude product was purified by column chromatography on aluminum oxide (activity II–III) with dichloromethane as an eluent to yield a deep-yellow powder (231 mg, 25%). 1H NMR (400 MHz, CDCl3): δ 7.95 (s, 3H), 7.80 (d, J=8.0 Hz, 6H), 7.61 (d, J=8.0 Hz, 6H), 6.56 (d, J=4.0 Hz, 6H), 6.19 (d, J=4.0 Hz, 6H), 2.47 (s, 18H); 13C NMR (100 MHz, CDCl3): δ 165.18, 146.78, 141.99, 137.88, 135.32, 132.12, 130.51, 128.89, 127.12, 116.54, 112.11, 105.97 and 18.21; HR-FAB-MS (m/z): [M]+ calculated for C57H48N6, 816.3940; found, 816.3924.
Synthesis of multi-layer N1
A 50-ml cylindrical glass vial (3.2 cm in diameter) was used as the reaction container. L1 was dissolved in dichloromethane to a concentration of 1.0 × 10−4 mol l−1, and 10 ml of the solution was poured into the vial. Then, pure water (10 ml) was layered gently onto the organic phase, which served as a buffer layer. After 2 h, aqueous zinc(II) acetate solution (0.1 mol l−1, 10 ml) was slowly added to the buffer layer. The reaction system was left undisturbed for 4 days to obtain an orange film at the liquid/liquid interface. The resulting multi-layer N1 could be deposited on various substrates.
Single-phase reactions between L1 and zinc(II) acetate
Two types of reaction were performed. Stoichiometric amounts of zinc(II) acetate dihydrate (4.9 mg, 0.022 mmol) and L1 (12.3 mg, 0.015 mmol) were dissolved in dichloromethane (5 ml) in a glass vial. The reaction mixture was allowed to stand at room temperature for 1 day. The resultant product was filtered and washed with dichloromethane, ethanol and water to yield a brown solid (Fig. 2f). In the other type of reaction, stoichiometric amounts of zinc(II) acetate dihydrate (5.1 mg, 0.023 mmol) and L1 (12.2 mg, 0.015 mmol) were dissolved in N,N-dimethylformamide (5 ml) in a glass vial. After sealing the vial tightly, the reaction mixture was heated at 105 °C for 1 day in an oil bath. After being cooled down to room temperature, the resultant product was filtered and washed with dichloromethane, N,N-dimethylformamide and water to obtain a brown solid (Fig. 2g).
Synthesis of single-layer or few-layer N1
A 50-ml glass vial (3.2 cm in diameter) was used as the reaction container. Aqueous zinc(II) acetate (0.05 mol l−1, 30 ml) was poured into the vial. A small amount of a dichloromethane solution of L1 was then sprinkled gently on the surface of the aqueous phase: single-layer N1 was synthesized using 5.0 μl of L1 solution (7.4 × 10−5 mol l−1), whereas 20 μl of L1 solution (7.4 × 10−5 mol l−1) was used for the few-layer nanosheet. After spontaneous evaporation of the organic solvent, the reaction system was left undisturbed, such that N1 was produced at the air/liquid interface. Single-layer or few-layer N1 was then transferred onto substrates using the Langmuir–Schäfer method.
Synthesis of mononuclear bis(dipyrrinato)zinc(II) complex M1
Zinc(II) acetate dihydrate (36.7 mg, 0.17 mol) and triethylamine (0.11 ml) were added to a dichloromethane solution (20 ml) of 2-((3,5-dimethyl-2H-pyrrol-2-ylidene)(2,6-dimethylphenyl)methyl)-3,5-dimethyl-1H-pyrrole (122 mg, 0.40 mmol), and the reaction mixture was stirred overnight at room temperature. Methanol (20 ml) was added to recrystallize the product as an orange solid (60.5 mg, 45%). 1H NMR (500 MHz, CDCl3): δ 7.21 (t, J=7.6 Hz, 2H), 7.11 (d, J=7.6 Hz, 4H), 5.91 (s, 4H), 2.16 (s, 12H), 2.04 (s, 12H) and 1.28 (s, 12H); 13C NMR (125 MHz, CDCl3): δ 156.07, 143.26, 143.06, 139.18, 135.95, 134.27, 127.98, 127.88, 119.74, 19.33, 16.13 and 14.70; HR-FAB-MS (m/z): [M]+ calculated for C42H46N4Zn, 670.3014; found, 670.3011.
Synthesis of mononuclear bis(dipyrrinato)zinc(II) complex M2
A solution of 2-methyl-5-((5-methyl-2H-pyrrol-2-ylidene)(phenyl)methyl)-1H-pyrrole (450 mg, 1.8 mmol) in methanol (10 ml) was poured into a suspension of anhydrous zinc(II) acetate (168 mg, 0.92 mmol) in methanol (3 ml). The mixture was stirred overnight at room temperature. The reaction mixture was filtered, and the resultant solid was washed with methanol. Filtration yielded a light orange solid (463 mg, 90%). 1H NMR (400 MHz, CDCl3): δ 7.50−7.39 (m, 10H), 6.55 (d, J=4.0 Hz, 2H), 6.18 (d, J=4.0 Hz, 2H) and 2.12 (s, 12H); 13C NMR (100 MHz, CDCl3): δ 159.44, 144.71, 139.52, 139.39, 133.22, 130.89, 128.17, 127.14, 117.10 and 16.62; HR-FAB-MS (m/z): [M]+ calculated for C42H46N4Zn, 558.1762; found, 558.1782.
Synthesis of a precursor for M3
To a dichloromethane (20 ml) solution of ethyl 3-(4-formylphenyl)propanoate (618 mg, 3.0 mmol) and 2-methylpyrrole (0.55 ml, 6.3 mmol), trifluoroacetic acid (10 μl) was added, under a nitrogen atmosphere, and the solution was stirred overnight at room temperature. Chloranil (749 mg, 3.0 mmol) was added and stirred for another 30 min. After evaporation of the solvent, the residue was passed through a short pad of aluminum oxide (activity II–III) using dichloromethane as an eluent. After evaporation of the solvent, the crude product was purified by column chromatography on aluminum oxide (activity II–III) with hexane/dichloromethane (1:1 v/v) as an eluent. The yellow band was collected and evaporated to yield ethyl 3-(4-((5-methyl-1H-pyrrol-2-yl)(5-methyl-2H-pyrrol-2-ylidene)methyl)phenyl)propanoate as an orange oil (146 mg, 14%). 1H NMR (500 MHz, CDCl3) δ 7.41 (d, J=8.2 Hz, 2H), 7.26 (d, J=8.2 Hz, 2H), 6.48 (d, J=3.8 Hz, 2H), 6.17 (d, J=3.8 Hz, 2H), 4.18 (q, J=7.3 Hz, 2H), 3.05 (t, J=7.9 Hz, 2H), 2.71 (t, J=7.9 Hz, 2H), 2.46 (s, 6H) and 1.28 (t, J=7.3 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 172.84, 153.67, 140.99, 139.91, 138.30, 135.49, 130.99, 128.96, 127.41, 117.35, 60.49, 35.81, 30.79, 16.29 and 14.27; HR-FAB-MS (m/z): [M]+ calculated for C22H24N2O2, 348.1829; found, 348.1838.
Synthesis of M3
Ethyl 3-(4-((5-methyl-1H-pyrrol-2-yl)(5-methyl-2H-pyrrol-2-ylidene)methyl)phenyl)propanoate (100 mg, 0.29 mmol) was added to tetrahydrofuran (30 ml) and water (5 ml), and the mixture was stirred at room temperature for 5 min. Then, an aqueous solution (10 ml) of NaOH (115 mg, 2.9 mmol) was added, and the reaction mixture was stirred overnight at room temperature. After adjusting the pH of the reaction mixture to 4 using aqueous hydrochloric acid (1.0 × 10−4 mol l−1), the mixture was poured into water (50 ml) and washed with ether (20 ml × 3). The water layer was then treated with a solution of anhydrous zinc(II) acetate (58 mg, 0.29 mmol) in water (3 ml). The mixture was extracted with dichloromethane and dried over sodium sulfate. The solvent was evaporated under a reduced pressure to yield a dark orange solid of M3 (3.0 mg, 3%). 1H NMR (500 MHz, dimethylsulfoxide-d6): δ 12.34 (bs, 2H), 7.34–7.30 (m, 8H), 6.33 (d, J=4.0 Hz, 4H), 6.20 (d, J=4.0 Hz, 4H), 2.91 (t, J=7.6 Hz, 4H), 2.61 (t, J=7.6 Hz, 4H) and 2.39 (s, 12H); 13C NMR (125 MHz, CDCl3) δ 158.99, 144.79, 139.06, 133.29, 130.90, 130.59, 127.60, 117.85, 31.16, 25.62 and 16.49 (two quaternary carbons are missing because of low solubility or overlaps with other peaks); HR-ESI-MS (m/z): [M-H+] calculated for C40H37N4O4Zn, 701.2106; found, 701.2122.
Pretreatments for substrates
HOPG was cleaved with a piece of cellophane tape to obtain a flat and clean surface just before use. Si(111) substrates were hydrophobized using HMDS. The procedure to form a HMDS/Si(111) surface is as follows. The Si(111) substrate covered with a natural SiO2 layer was sonicated in acetone (10 min), 2-propanol (15 min × 2), water (15 min) and ethanol (10 min). After rinsing the substrate in ethanol a couple of times, the substrate was immersed in an ethanol solution of HMDS (1 v/v%) for 1 h. The substrate was then washed with ethanol several times before being annealed at 130 °C for 30 min. The modified substrate was then sonicated in ethanol (15 min) and water (10 min) and was stored in water. Just before use, HMDS/Si(111) was sonicated in 2-propanol (15 min) and water (15 min), which was dried in vacuo overnight. ITO (on glass), quartz and SnO2 (on ITO-covered glass, 5 Ω sq−1) substrates were sonicated in acetone (10 min × 2), water (10 min × 2) and nonionic detergent in water (30 min). Then, the substrate was washed with water until the bubbles of the detergent disappeared before being sonicated in water (10 min × 3) and ethanol (10 min × 2). The cleaned substrate was stored in water, and dried by nitrogen blow just before use.
Layering of single-layer N1
A Langmuir–Blodgett trough (KSV 2000, KSV NIMA) was used as a reaction container. Before a zinc(II) acetate aqueous solution (0.05 mol l−1) was poured into the trough as a subphase, the trough was washed with ethanol. The surface of the aqueous solution was cleaned by suctioning the surface of the subphase several times on compressing the trough barrier. Then, a dichloromethane solution of L1 (7.4 × 10−5 mol l−1, 27.7 μl) was dropped gently onto the surface of the subphase. Under this condition, the ideal coverage of single-layer N1 reached 90% of the trough area. After spontaneous evaporation of the organic solvent, the reaction system was left undisturbed for 4 h, such that single-layer N1 was produced at the air/liquid interface. The trough was then compressed to yield a trough area of 69.8 cm2 (90% of the initial trough area) at a surface pressure of 0.04 mN m−1. Under these conditions, single-layer N1 was transferred onto a quartz substrate via the Langmuir–Schäfer method. The trough was then shrunk by the same area as the substrate, and the next transfer was conducted. This process was repeated to layer single-layer N1.
Guest inclusion
Multi-layer N1 (thickness: 700 nm) was deposited on a quartz or ITO substrate. The modified substrate was immersed in a dichloromethane solution of Rhodamine B (5.0 × 10−5 mol l−1) for 15 h and was then rinsed with dichloromethane to yield Rhodamine B-encapsulated multi-layer N1.
Analyses for N1
XPS was conducted using a PHI 5,000 VersaProbe (Ulvac-Phi, Inc.). Al Kα (15 kV, 25 W) was used as the X-ray source, and the beam was focused on a 100-μm2 area. The spectra were analysed with MultiPak Software and standardized using the C 1s peak at 284.6 eV. AFM and STM were performed using an Agilent Technologies 5,500 scanning probe microscope under an ambient condition. AFM was performed in high-amplitude mode (tapping mode) with a silicon cantilever PPP-NCL (Nano World). The probe for STM (Pt-Ir alloy, 4:1, 0.25 mm in diameter) was cut from a wire using a nipper to obtain a sharp edge. An optical microscope image was taken using a VHX-100 (Keyence Corporation). A field-emission scanning electron microscopic image was collected using a JEOL JSM-7400FNT. Transmission electron microscopy images/SAED patterns were recorded at accelerating voltage of 75 kV using a Hitachi HF-2000 equipped with an AMT-CCD camera. The sample was prepared by depositing multi-layer N1 (thickness: 700 nm) on a carbon film supported by a copper grid (ELS-C10, stem Co., Ltd) directly from the liquid/liquid interface. To acquire electron diffractions, we focused on the edge of multi-layer N1. To reproduce the obtained SAED pattern, three-dimensional structures of multi-layer N1, which comprise piles of single-layer N1, were considered (given as a Supplementary Information and shown in Supplementary Fig. 8). Here we treated AA-, AB- and ABC-stack models, which are often encountered in layered materials59,60. The three-dimensional lattice was optimized at the molecular mechanics level of theory with the UFF VALBOND 1.1 force field on an Accelrys Cerius2 ver3.1 program package. The unit cell was assumed to be trigonal such that the α, β, and γ angles were constrained to be 90°, 90°, and 120°, respectively. First, we performed a calculation on the AA-stacking structure with its molecular geometries and cell lengths being fully optimized. Initial structures for the AB- and ABC-stack models were constructed from the optimized AA-stack one by giving parallel displacement to the B and/or C layers. The SAED patterns were simulated by implementing CrystalMaker 2.6.3 and SingleCrystal 2.3 (CrystalMaker Software Ltd) (Supplementary Fig. 7b–d). Ultraviolet/visible absorption spectra were recorded on a JASCO V-570 spectrometer in transmission mode. A quartz or SnO2 substrate modified with N1 was set vertical to the probe light. Fluorescence and excitation spectra were recorded on a HITACHI F-4500 fluorospectrometer.
Photoelectric conversion
N1 with thicknesses ≤155 layers was deposited on a transparent SnO2 electrode using the repeated Langmuir–Schäfer procedure shown in Fig. 6b,c. On the other hand, N1 with thicknesses of >155 layers was fabricated directly using the liquid/liquid interfacial synthesis (Fig. 2a) and deposited on the electrode. Before photoelectric conversion, each N1 physisorbed on a transparent SnO2 electrode was always subjected to ultraviolet/visible spectroscopy, acquiring spectra from four different positions. This series of measurements allowed us to ensure the uniformity of N1 and to quantify the thickness of N1 using the average absorbance at 500 nm and that of single-layer N1 (0.00101 from the slope of Fig. 6c). The average absorbance at 500 nm was also used in calculating the quantum yield of photoelectric conversion (vide infra). The modified SnO2 electrode was used as a working electrode (photoanode). Silver and platinum wires were employed as reference and counter electrodes, respectively. The resulting three-electrode system was immersed in a homemade photoelectrochemical cell (Supplementary Fig. 10), which was filled with an acetonitrile solution of tetra-n-butylammonium perchlorate (0.1 mol l−1) containing TEOA (0.05 mol l−1) as a sacrificial donor reagent. The cell was sealed and deoxygenized by argon bubbling for 30 min. Monochromatic light for the action spectrum shown in Fig. 7b (400–600 nm in every 20 nm) was extracted from a xenon lamp (MAX–302, Asahi Spectra Co., Ltd), the photon flux of which was monochromated by a monochromator (CT-10, JASCO Corporation). For the other experiments, 500-nm light was used exclusively, which was provided by the xenon lamp equipped with a band-pass filter. The active area of the electrode was 0.264 cm2, which was determined by a fluorocarbon rubber o-ring (Supplementary Fig. 10). The electrode potential and photocurrent acquisition of the photoelectric conversion system were controlled using an electrochemical analyser (ALS 750A, BAS Inc.). The photoanode was fixed at 0.0 V versus the silver reference electrode for the data shown in Fig. 7a–d and 0.15 V for the other data, including those for M2 and M3. At both potentials, no anodic current induced by the direct oxidation of TEOA in the dark was observed. The quantum yield of photoelectric conversion, φ, was calculated using equation (1):

where ne is the mole of electrons that flows in the circuit per unit time (in mol s−1) and np is the mole of photons absorbed by the sensitizer per unit time (in mol s−1). ne and np were calculated using equations (2) and (3):


where i is the current flow (in A), F is the Faraday constant (9.65 × 104 C mol−1), W is the photon flux of incident light (in J s−1), λ is the wavelength of the irradiated light (5.00 × 10−7 m), A is the absorbance at the irradiated wavelength, NA is the Avogadro constant (6.02 × 1023 mol−1), h is the Planck constant (6.63 × 10−34 Js) and c is the velocity of light (3.00 × 108 m s−1). A representative data set for the determination of φ for N1 is shown in Supplementary Fig. 11. i was calculated using equation (4):

where iL is the average light current for the first cycle (10 s) and iD is the average dark current just before the illumination of light. A photon counter (8230E and 82311B, ADC Corporation) was employed for the quantification of W. For every sample, W was measured independently. A typical value for W was ~0.20 mW. When i was below the measurable level (<1.0 nA), W was increased to 1.0–2.0 mW such that the photocurrent signal was amplified. For referential mononuclear complex M2, film formation was conducted as follows: two droplets from a disposable Pasteur pipette of an acetonitrile solution of M2 (1.86 × 10−5 mol l−1) were dripped onto a SnO2 substrate (~0.8 cm2). The substrate was then dried under vacuum for 10 min to allow the solvent to evaporate. The modified SnO2 electrode was subjected to photoelectric conversion, following the procedure for N1 except that aqueous 0.1 mol l−1 sodium sulfate was used as an electrolyte solution; this change prevented the M2 film from redissolution. Referential mononuclear complex M3 was immobilized on a SnO2 electrode through the carboxy group using the self-assembled monolayer procedure61. A SnO2 substrate was immersed in a dimethylsulfoxide solution of M3 (1.96 × 10−3 mol l−1) for 1 day at room temperature, and the decorated substrate was rinsed with dimethylsulfoxide and dried by an argon blow. The modified SnO2 electrode was subjected to photoelectric conversion using the same procedure as N1.
Additional information
How to cite this article: Sakamoto, R. et al. A photofunctional bottom-up bis(dipyrrinato)zinc(II) complex nanosheet. Nat. Commun. 6:6713 doi: 10.1038/ncomms7713 (2015).
References
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004) .
Geim, A. K. & Novoselov, K. S. The rise of graphene. Nat. Mater. 6, 183–191 (2007) .
Lin, Y.-M. et al. 100-GHz transistors from wafer-scale epitaxial graphene. Science 327, 662 (2010) .
Bonaccorso, F., Sun, Z., Hasan, T. & Ferrari, A. C. Graphene photonics and optoelectronics. Nat. Photonics 4, 611–622 (2010) .
Son, Y.-W., Cohen, M. L. & Louie, S. G. Half-metallic graphene nanoribbons. Nature 444, 347–349 (2006) .
Maassen, J., Ji, W. & Guo, H. Graphene spintronics: the role of ferromagnetic electrodes. Nano Lett. 11, 151–155 (2011) .
Sasaki, T., Watanabe, M., Hashizume, H., Yamada, H. & Nakazawa, H. Macromolecule-like aspects for a colloidal suspension of an exfoliated titanate. Pairwise association of nanosheets and dynamic reassembling process initiated from it. J. Am. Chem. Soc. 118, 8329–8335 (1996) .
Li, B.-W. et al. Engineered interfaces of artificial perovskite oxide superlattices via nanosheet deposition process. ACS Nano 4, 6673–6680 (2010) .
Umar, A. & Hahn, Y. B. ZnO nanosheet networks and hexagonal nanodiscs grown on silicon substrate: growth mechanism and structural and optical properties. Nanotechnology 17, 2174–2180 (2006) .
Sakai, N., Ebina, Y., Takada, K. & Sasaki, T. Electronic band structure of titania semiconductor nanosheets revealed by electrochemical and photoelectrochemical studies. J. Am. Chem. Soc. 126, 5851–5858 (2004) .
Muramatu, M. et al. Fabrication of densely packed titania nanosheet films on solid surface by use of Langmuir-Blodgett deposition method without amphiphilic additives. Langmuir 21, 6590–6595 (2005) .
Coleman, J. N. et al. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 331, 568–571 (2011) .
Splendiani, A. et al. Emerging photoluminescence in monolayer MoS2 . Nano Lett. 10, 1271–1275 (2010) .
Lee, H. S. et al. MoS2 nanosheets for top-gate nonvolatile memory transistor channel. Small 8, 3111–3115 (2012) .
Zeng, Z. et al. Single-layer semiconducting nanosheets: high-yield preparation and device fabrication. Angew. Chem. Int. Ed. 50, 11093–11097 (2011) .
Ma, R., Liu, Z., Li, L., Iyi, N. & Sasaki, T. Exfoliating layered double hydroxides in formamide: a method to obtain positively charged nanosheets. J. Mater. Chem. 16, 3809–3813 (2006) .
Ma, R. et al. Synthesis and exfoliation of Co2+-Fe3+ layered double hydroxides: an innovative topochemical approach. J. Am. Chem. Soc. 129, 5257–5263 (2007) .
Ida, S., Shiga, D., Koinuma, M. & Matsumoto, Y. Synthesis of hexagonal nickel hydroxide nanosheets by exfoliation of layered nickel hydroxide intercalated with dodecyl sulfate ions. J. Am. Chem. Soc. 130, 14038–14039 (2008) .
Yan, D. et al. Ordered poly(p-phenylene)/layered double hydroxide ultrathin films with blue luminescence by layer-by-layer assembly. Angew. Chem. Int. Ed. 48, 3073–3076 (2009) .
Yan, D. et al. Reversibly thermochromic, fluorescent ultrathin films with a supramolecular architecture. Angew. Chem. Int. Ed. 50, 720–723 (2011) .
Sakamoto, J., van Heijst, J., Lukin, O. & Schlüter, A. D. Two-dimensional polymers: just a dream of synthetic chemists? Angew. Chem. Int. Ed. 48, 1030–1069 (2009) .
Kissel, P. et al. A two-dimensional polymer prepared by organic synthesis. Nat. Chem 4, 287–291 (2012) .
Payamyar, P. et al. Synthesis of a covalent monolayer sheet by photochemical anthracene dimerization at the air/water interface and its mechanical characterization by AFM indentation. Adv. Mater. 26, 2052–2058 (2014) .
Kory, M. J. et al. Gram-scale synthesis of two-dimensional polymer crystals and their structure analysis by X-ray diffraction. Nat. Chem. 6, 779–784 (2014) .
Kissel, P., Murray, D. J., Wulftange, W. J., Catalano, V. J. & King, B. T. A nanoporous two–dimensional polymer by single–crystal–to–single–crystal photopolymerization. Nat. Chem. 6, 774–778 (2014) .
Bauer, T. et al. Synthesis of free standing, monolayered organometallic sheets at the air/water interface. Angew. Chem. Int. Ed. 50, 7879–7884 (2011) .
Zheng, Z. et al. Synthesis of two-dimensional analogues of copolymers by site-to-site transmetalation of organometallic monolayer sheets. J. Am. Chem. Soc. 136, 6103–6110 (2014) .
Schrettl, S. et al. Functional carbon nanosheets prepared from hexayne amphiphile monolayers at room temperature. Nat. Chem. 6, 468–476 (2014) .
Makiura, R. et al. Surface nano-architecture of a metal-organic framework. Nat. Mater. 9, 565–571 (2010) .
Motoyama, S., Makiura, R., Sakata, O. & Kitagawa, H. Highly crystalline nanofilm by layering of porphyrin metal-organic framework sheets. J. Am. Chem. Soc. 133, 5640–5643 (2011) .
Zwaneveld, N. A. A. et al. Organized formation of 2D extended covalent organic frameworks at surfaces. J. Am. Chem. Soc. 130, 6678–6679 (2008) .
Tanoue, R. et al. Thermodynamically controlled self-assembly of covalent nanoarchitectures in aqueous solution. ACS Nano 5, 3923–3929 (2011) .
Dienstmaier, J. F. et al. Synthesis of well-ordered COF monolayers: surface growth of nanocrystalline precursors versus direct on-surface polycondensation. ACS Nano 5, 9737–9745 (2011) .
Liu, X.-H. et al. On-surface synthesis of single-layered two-dimensional covalent organic frameworks via solid-vapor interface reactions. J. Am. Chem. Soc. 135, 10470–10474 (2013) .
Grill, L. et al. Nano-architectures by covalent assembly of molecular building blocks. Nat. Nanotech. 2, 687–691 (2007) .
Gutzler, R. et al. Surface mediated synthesis of 2D covalent organic frameworks: 1,3,5-tris(4-bromophenyl)benzene on graphite(001), Cu(111), and Ag(110). Chem. Commun. 4456–4458 (2009) .
Abel, M., Clair, S., Ourdjini, O., Mossayan, M. & Porte, L. Single-layer of polymeric Fe-phthalocyanine: an organometallic sheet on metal and thin insulating film. J. Am. Chem. Soc. 133, 1203–1205 (2011) .
Li, Y. et al. Coordination and metalation bifunctionality of Cu with 5,10,15,20-tetra(4-pyridyl)porphyrin: toward a mixed-valence two-dimensional coordination network. J. Am. Chem. Soc. 134, 6401–6408 (2012) .
Sirtl, T. et al. Control of intermolecular bonds by deposition rates at room temperature: hydrogen bonds versus metal coordination in trinitrile monolayers. J. Am. Chem. Soc. 135, 691–695 (2013) .
Berlanga, I. et al. Delamination of layered covalent organic frameworks. Small 7, 1207–1211 (2011) .
Berlanga, I., Rubén, M.-B. & Zamora, F. Tuning delamination of layered covalent organic frameworks through structural design. Chem. Commun. 48, 7976–7978 (2012) .
Bunck, D. N. & Dichtel, W. R. Bulk synthesis of exfoliated two-dimensional polymers using hydrazone-linked covalent organic frameworks. J. Am. Chem. Soc. 135, 14952–14955 (2013) .
Amo-Ochoa, P. et al. Single layers of a multifunctional laminar Cu(I,II) coordination polymer. Chem. Commun. 46, 3262–3264 (2010) .
Li, P.-Z., Maeda, Y. & Xu, Q. Top-down fabrication of crystalline metal-organic framework nanosheet. Chem. Commun. 47, 8436–8438 (2011) .
Tan, J.-C., Saines, P. J., Bithell, E. G. & Cheetham, A. K. Hybrid nanosheets of an inorganic-organic framework material: facile synthesis, structure, and elastic properties. ACS Nano 6, 615–621 (2012) .
Xu, G., Yamada, T., Otsubo, K., Sakaida, S. & Kitagawa, H. Facile ‘modular assembly’ for fast construction of a highly oriented crystalline MOF nanofilm. J. Am. Chem. Soc. 134, 16524–16527 (2012) .
Kambe, T. et al. π-conjugated nickel bisdithiolene complex nanosheet. J. Am. Chem. Soc. 135, 2462–2465 (2013) .
Hoshiko, K., Kambe, T., Sakamoto, R., Takada, K. & Nishihara, H. Fabrication of dense and multi-layered films of the nickel bis(dithiolene) nanosheet by means of the Langmuir-Schäfer method. Chem. Lett. 43, 252–253 (2014) .
Kambe, T. et al. Redox control and high conductivity of nickel bis(dithiolene) complex π-nanosheet: A potential organic two-dimensional topological insulator. J. Am. Chem. Soc. 136, 14357–14360 (2014) .
Wood, T. E. & Thompson, A. Advances in the chemistry of dipyrrins and their complexes. Chem. Rev. 107, 1831–1861 (2007) .
Maeda, H. et al. Nanoscale spherical architectures fabricated by metal coordination of multiple dipyrrin moieties. J. Am. Chem. Soc. 128, 10024–10025 (2006) .
Sazanovich, I. V. et al. Structural control of the excited-state dynamics of bis(dipyrrinato)zinc complexes: self-assembling chromophores for light-harvesting architectures. J. Am. Chem. Soc. 126, 2664–2665 (2004) .
Kusaka, S., Sakamoto, R., Kitagawa, Y., Okumura, M. & Nishihara, H. An extremely bright heteroleptic bis(dipyrrinato)zinc(II) complex. Chem. Asian J. 7, 907–910 (2012) .
Tsuchiya, M. et al. Asymmetric dinuclear bis(dipyrrinato)zinc(II) complexes: Broad absorption and unidirectional quantitative exciton transmission. Chem. Commun. 50, 5881–5883 (2014) .
Karweik, D. H. & Winograd, N. Nitrogen charge distributions in free-base porphyrins, metalloporphyrins, and their reduced analogs observed by X-ray photoelectron spectroscopy. Inorg. Chem. 15, 2336–2342 (1976) .
Wang, Z. et al. Phenanthro[9,10-d]imidazole as a new building block for blue light emitting materials. J. Mater. Chem. 21, 5451–5456 (2011) .
Micheal, M.-C. L. & Gregory, C. F. Cu(I)/bis(azaferrocene)-catalyzed enantioselective synthesis of β-lactams via couplings of alkynes with nitrones. J. Am. Chem. Soc. 124, 4572–4573 (2002) .
Qin, W., Baruah, M., Van der Auweraer, M., De Schryver, F. C. & Boens, N. Photophysical properties of borondipyrromethene analogues in solution. J. Phys. Chem. A 109, 7371–7384 (2005) .
Spitler, E. L. & Dichtel, W. R. Lewis acid-catalysed formation of two-dimensional phthalocyanine covalent organic frameworks. Nat. Chem. 2, 672–677 (2010) .
Ping, J. & Fuhrer, M. S. Layer number and stacking sequence imaging of few-layer graphene by transmission electron microscopy. Nano Lett. 12, 4635–4641 (2012) .
Nagaoka, S., Horiuchi, K., Shikishima, M. & Nakajima, A. Physical properties of aliphatic monolayer on indium-tin oxide and SnO2(110) relevant to thermal stability of soft-landed Cr(benzene)2 . J. Phys. Chem. C 115, 24215–24220 (2011) .
Acknowledgements
We acknowledge Grants-in-Aid from MEXT of Japan (Nos. 24750054, 25107510, 26708005, 26107510, 26620039, 21108002, 26220801, 26248017, 26110505, 26410093, areas 2107 (Coordination Programming), 2406 (All Nippon Artificial Photosynthesis Project for Living Earth), 2506 (Science of Atomic Layers), and 2509 (Molecular Architectonics)). R.S. is grateful to Ogasawara Foundation for the Promotion of Science and Engineering, Noguchi Institute, Tokuyama Science Foundation, Asahi Glass Foundation, The Murata Science Foundation, Iketani Science and Technology Foundation, The Japan Prize Foundation, Kao Foundation for Arts and Sciences, Japan Association for Chemical Innovation, The MIKIYA Science and Technology Foundation, Yazaki Memorial Foundation for Science and Technology, Shorai Foundation for Science and Technology, The Kurata Memorial Hitachi Science and Technology Foundation, Kumagai Foundation for Science and Technology, and The Japan Science Society for financial supports. W.-Y.W. thanks the Hong Kong Research Grants Council (HKBU 12302114), Areas of Excellence Scheme of HKSAR (AoE/P-03/08), National Natural Science Foundation of China (Project Nos. 51373145 and 91333206), Science, Technology, and Innovation Committee of Shenzhen Municipality (JCYJ20140419130507116) and Hong Kong Baptist University (FRG2/13-14/083) for the financial support. This work was also supported by the Partner State Key Laboratory of Environmental and Biological Analysis (SKLP-14-15-P011) and Strategic Development Fund of HKBU. K.H. acknowledges Fostering program of human resources in chemistry conducted by Japan Chemical Industry Association. M.T. is grateful to Advanced Leading Graduate Course for Photon Science (ALPS) and the JSPS fellowship for young scientists. Y.K. expresses gratitude to Professor Mitsutaka Okumura for valuable supports and discussion on the molecular mechanics calculation. We also thank the Research Hub Advanced Nano Characterization (The University of Tokyo) and Dr Tetsuro Kusamoto (The University of Tokyo) for XPS and electrospray ionization time-of-flight spectrometry measurements, respectively.
Author information
Authors and Affiliations
Contributions
R.S. conceived and directed the project. R.S., H.N., K.H. and W.-Y.W. initiated the project. S.K., Q.L. and W.-Y.W. synthesized ligand L1. K.H., Q.L. and T.N. performed the fabrication of N1 and M1. T.N. and M.T. synthesized M2 and M3, respectively. Y.K. optimized the three-dimensional lattice of multi-layer N1 at the molecular mechanics level of theory. K.H. conducted the characterization of N1. T.Y. and K.H. investigated the photoelectric conversion of N1, M2 and M3. R.S. and K.H. co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-14 and Supplementary References (PDF 14614 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Sakamoto, R., Hoshiko, K., Liu, Q. et al. A photofunctional bottom-up bis(dipyrrinato)zinc(II) complex nanosheet. Nat Commun 6, 6713 (2015). https://doi.org/10.1038/ncomms7713
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms7713
This article is cited by
-
Metal-organic layers: Preparation and applications
Science China Materials (2023)
-
A Bis(terpyridine)nickel(II)-Based Coordination Nanosheet: A Redox-Active Material with Flexibility and Transparency
Journal of Inorganic and Organometallic Polymers and Materials (2023)
-
Correlation-induced magnetism in substrate-supported 2D metal-organic frameworks
npj Computational Materials (2022)
-
Emerging porous nanosheets: From fundamental synthesis to promising applications
Nano Research (2021)
-
Unimolecularly thick monosheets of vinyl polymers fabricated in metal–organic frameworks
Nature Communications (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.