Abstract
With current research efforts shifting towards the 4d and 5d transition metal oxides, understanding the evolution of the electronic and magnetic structure as one moves away from 3d materials is of critical importance. Here we perform X-ray spectroscopy and electronic structure calculations on A-site-ordered perovskites with Cu in the A-site and the B-sites descending along the ninth group of the periodic table to elucidate the emerging properties as d-orbitals change from partially filled 3d to 4d to 5d. The results show that when descending from Co to Ir, the charge transfers from the cuprate-like Zhang-Rice state on Cu to the t2g orbital of the B site. As the Cu d-orbital occupation approaches the Cu2+ limit, a mixed valence state in CaCu3Rh4O12 and heavy fermion state in CaCu3Ir4O12 are obtained. The investigated d-electron compounds are mapped onto the Doniach phase diagram of the competing RKKY and Kondo interactions developed for the f-electron systems.
Similar content being viewed by others
Introduction
Transition metal (TM) oxides host a diversity of fascinating phenomena1,2,3,4,5,6. Several possible formal oxidation states of the TM ions coupled with the innate ability to stabilize those states by structural network of oxygens give rise to a striking change in the TM–O orbital hybridization W, electron–electron correlations U and the charge transfer energy Δ7; the subtle competition between W, U and Δ across the 3d group of the periodic table is then responsible for a vast landscape of interesting magnetic and electronic ground states1,4,8. In traversing the periodic table within groups of the 3d→4d→5d TM blocks, the additional degree of freedom, the spin–orbit (SO) interaction, λ gets activated and is predicted to foster a multitude of exotic electronic and topological phases of correlated matter9,10,11,12,13,14,15.
Although the vast majority of these compounds contain TM ions at the B-site of the ABO3 perovskite structure, it is now possible to synthesize a new family of compounds with formula unit  , Fig. 1a, where the perovskite A-site is partially occupied by a TM ion, labelled A′. Among this class of materials, (ACu3)B4O12 with Cu in the A′ site have garnered considerable attention because of the presence of CuO4 planes. Structurally, this family of compounds consists of two different magnetically active sublattices of TM ions: BO6 octahedral units (forming a three-dimensional octahedral network as in the typical ABO3 perovskite lattice) and the planar CuO4 units coupled with BO6 units via an apical oxygen (see Fig. 1a). Depending on the choice of B-site ion, the materials exhibit exciting properties including giant dielectric constant (B=Ti), exotic ferromagnetism (B=Ge, Sn, Fe), valence fluctuation (B=V), Mott physics (B=Ru) and inter-site charge order (B=Fe) to name a few16,17,18,19,20,21,22. They are also of particular interest owing to the preservation of the cubic lattice symmetry
, Fig. 1a, where the perovskite A-site is partially occupied by a TM ion, labelled A′. Among this class of materials, (ACu3)B4O12 with Cu in the A′ site have garnered considerable attention because of the presence of CuO4 planes. Structurally, this family of compounds consists of two different magnetically active sublattices of TM ions: BO6 octahedral units (forming a three-dimensional octahedral network as in the typical ABO3 perovskite lattice) and the planar CuO4 units coupled with BO6 units via an apical oxygen (see Fig. 1a). Depending on the choice of B-site ion, the materials exhibit exciting properties including giant dielectric constant (B=Ti), exotic ferromagnetism (B=Ge, Sn, Fe), valence fluctuation (B=V), Mott physics (B=Ru) and inter-site charge order (B=Fe) to name a few16,17,18,19,20,21,22. They are also of particular interest owing to the preservation of the cubic lattice symmetry  , despite large variations of the B-site ion including utilizing different TM series (3d or 4d or 5d) of the periodic table23. This provides a unique platform to investigate the emergence of the electronic and magnetic states; for example, in recent work on (CaCu3)B4O12 (B=Cr, Co), it has been demonstrated that the Zhang-Rice quantum state (essential for hole doped high-Tc superconductivity) can be realized in these compounds, despite the lack of the superconducting ground state24,25,26.
, despite large variations of the B-site ion including utilizing different TM series (3d or 4d or 5d) of the periodic table23. This provides a unique platform to investigate the emergence of the electronic and magnetic states; for example, in recent work on (CaCu3)B4O12 (B=Cr, Co), it has been demonstrated that the Zhang-Rice quantum state (essential for hole doped high-Tc superconductivity) can be realized in these compounds, despite the lack of the superconducting ground state24,25,26.
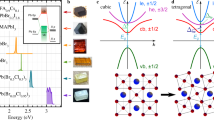
(a) Crystal structure of A-site-ordered perovskites. Connection between CuO4 planes and IrO6 octahedra shown in the bottom right corner. (b) Temperature-dependent transport data for the B=Co, Rh, Ir samples displaying the anomalous behaviour for CCCIrO. CCCoO data taken from ref. 22. CCIrO data taken from ref. 27.
Very recently, Cheng et al. showed that this class of materials displays a crossover between the magnetic insulating and paramagnetic metallic states, depending on the Cu–O and B–O bond lengths27. It was further suggested that the CaCu3Ir4O12 (CCIrO) compound, with the bond length within the crossover region, possesses anomalous electronic and magnetic properties arising presumably due to the interaction between the localized Cu and itinerant Ir states23. However, the mechanism by which the electronic structure transforms to create this emergent behaviour in CCIrO is not microscopically understood. In order to shed light on this interesting aspect, three isostructural and isoelectronic compounds whose B-site spans the ninth group of the periodic table, (CaCu3)B4O12 (B=Co, Rh, Ir), were synthesized. Within the proposed phase diagram of Cheng et al., B=Co occupies the paramagnetic metallic state, whereas CCIrO is at the crossover region as reflected for instance in anomalous d.c. transport properties shown in Fig. 1b (refs 22, 27).
In this paper, we investigate the electronic and magnetic structure of this new class of materials by a combination of resonant soft and hard X-ray absorption spectroscopy (XAS) on the Cu L-edge, O K-edge, and B-site L- and K-edges. Complementary first-principles calculations corroborate the experimental findings, providing microscopic understanding. Our results reveal that the unusual physical properties of these compounds are microscopically controlled by the degree of  orbital occupancy and the strength of the B–O covalency.
orbital occupancy and the strength of the B–O covalency.
Results
Spectroscopic results
First, we discuss the evolution of the electronic structure of Cu. The Cu L3-edge XAS are presented in Fig. 2a (total fluorescence yield (TFY) available in supplementary Fig. 1). In accord with the previously reported results24 for CaCu3Co4O12 (CCCoO), the main absorption line at 931.4 eV arises due to absorption from the  transition, where
transition, where  stands for a ligand hole whereas
stands for a ligand hole whereas  indicates a hole in the Cu 2p core states. The low-energy shoulder around 930 eV arises from the
indicates a hole in the Cu 2p core states. The low-energy shoulder around 930 eV arises from the  transition; the d9 transition accounting for 10% of the total peak area, indicating that there is a large Cu–O hybridization present in CCCoO. The line shape of the Cu L3 absorption edge for CaCu3Rh4O12 (CCRhO), containing the 4d element Rh at the B-site, also contains a lesser and yet still significant
transition; the d9 transition accounting for 10% of the total peak area, indicating that there is a large Cu–O hybridization present in CCCoO. The line shape of the Cu L3 absorption edge for CaCu3Rh4O12 (CCRhO), containing the 4d element Rh at the B-site, also contains a lesser and yet still significant  contribution, giving a Cu valence of ~2.6+. The transport behaviour for this compound is quite similar to CCCO and points to CCRhO also being paramagnetic metallic (Fig. 1b). On the other hand, for CCIrO, the
contribution, giving a Cu valence of ~2.6+. The transport behaviour for this compound is quite similar to CCCO and points to CCRhO also being paramagnetic metallic (Fig. 1b). On the other hand, for CCIrO, the  state is no longer present and the L3 line shape is almost analogous to that recorded from purely ionic d9 Cu2+ charge state, agreeing with an earlier report utilizing electron energy loss spectroscopy2,3,23,28,29,30. This implies that the hole is no longer interacting with the
state is no longer present and the L3 line shape is almost analogous to that recorded from purely ionic d9 Cu2+ charge state, agreeing with an earlier report utilizing electron energy loss spectroscopy2,3,23,28,29,30. This implies that the hole is no longer interacting with the  final state, showing a significant reduction of the hole contribution to the hybridized orbital between Cu and O. Furthermore, the small multiplet split peak seen around ~940 eV for all Cu L3-edge spectra is due to a transition from the metastable 3d8 (Cu3+) to the
final state, showing a significant reduction of the hole contribution to the hybridized orbital between Cu and O. Furthermore, the small multiplet split peak seen around ~940 eV for all Cu L3-edge spectra is due to a transition from the metastable 3d8 (Cu3+) to the  state; the small decrease in this 3d8 peak spectral weight in going from Co to Ir also indicates movement towards the ionic 3d9 state of Cu29,30,31. Taken as a whole, the Cu L3-edge data directly illustrate that in the presence of 4d or 5d orbitals, it is energetically more favourable to transfer the hole on to the B-site instead of stabilizing the Cu3+ state, suggesting a strong change in covalency, as tabulated in Table 1.
state; the small decrease in this 3d8 peak spectral weight in going from Co to Ir also indicates movement towards the ionic 3d9 state of Cu29,30,31. Taken as a whole, the Cu L3-edge data directly illustrate that in the presence of 4d or 5d orbitals, it is energetically more favourable to transfer the hole on to the B-site instead of stabilizing the Cu3+ state, suggesting a strong change in covalency, as tabulated in Table 1.
(a) Soft XAS on the Cu L-edge for all samples showing the changing Cu valence. The dotted line is the spectrum before the subtraction of the impurity peak (note that CCCoO TFY data and explanation of impurity peak originally given in ref. 24). The dashed line indicates the energy of the d9 peak. (b) XAS on the O K-edge showing both the reduction of the pre-peak on O and the shifting of the O 2p–Cu 3d and O 2p–B-site d hybridized orbitals. The dashed line here indicates the energy of the O pre-peak associated with a doped hole.
In the past, the electronic structure of Cu has been extensively studied in the context of high-Tc superconducting cuprates, where it was also found that the reduction of ligand hole weight on Cu causes a decrease of the pre-peak intensity around 528 eV in the O K-edge XAS spectrum32,33,34,35. To track the movement of the hole in the present series of samples, we obtained O K-edge XAS spectra shown in Fig. 2b, with the post-edge normalized to 1 (at 540 eV). As immediately seen, the decrease of the relative intensity of the pre-peak indeed scales with the reduction of the ligand hole on Cu, implying a marked change in both bandwidth, W, and the charge transfer gap, Δ. The decrease of the pre-peak intensity can be rationalized in terms of a decreasing availability of empty states as the hole concentration on O decreases in moving from Co to Rh to Ir. Although the high-energy shoulder on the Cu L3 peak disappears entirely for CCIrO, the oxygen pre-peak does not, which indicates the hybridization between the B-site and O is also significant, as Cu2+ will not contribute to the pre-peak. The results of the O K-edge spectroscopy are thus in excellent agreement with the Cu L-edge observations. Another interesting observation is that the energy separation between the pre-peak and the peak around 530 eV increases from Co to Ir. The shift towards higher energy will be discussed in detail in the theory section and is attributed to the gradually increasing separation of the B-site bands (CCCoO) and both the B-site and Cu bands (CCRhO and CCIrO) from the O 2p band.
The movement of the hole away from Cu naturally implies a change in valency of the B-site ion. To verify this proposition and to corroborate the previous findings, we performed XAS on the L- and K-edges of Ir and Rh, respectively (Co was measured previously and found to be in the ~ 3.25+ state, after subtraction of an impurity peak24). Moving to the 4d compound, Rh K-edge XAS spectra have been simultaneously collected from the CCRhO sample and a standard SrRhO3 reference sample. Although the line shape from CCRhO shares similarities with SrRhO3 (Rh4+), the difference in energy at 80% of the normalized absorption was found to be ~0.68 eV lower relative to SrRhO3, Fig. 3a (ref. 36). Based on this shift, and the shift of ~1.9 eV between Rh3+ and Rh4+ (see Supplementary Fig. 2), we conclude that the Rh valence state is indeed strongly mixed between 3+ and 4+, with a value of 3.64+ (±0.1). In conjunction with the Cu and O soft XAS, the entire Rh data set strongly supports the notion that the hole still largely resides on the O anion, but spreads to the hybridized orbital with Rh. Finally, the Ir L3 edge (2p3/2→5d transition) recorded from CCIrO and SrIr(4+)O3 is shown in Fig. 3b. As seen, the remarkably similar line shape and the energy peak position both confirm that Ir is in the 4+ state. The L3 to L2 branching ratio was found to be 3.79 (analysis shown in Supplementary Fig. 2)9,11. This value is similar to that found in several other iridate compounds and signifies a large SO coupling (SOC) as expected for a 5d compound9,11,37,38. Overall, the Ir L-edge data are in excellent agreement with the Cu L-edge data stating the d9 Cu2+ ground state, with a much smaller d8 contribution compared with the Rh and Co compounds; thus, in the CCIrO compound, the hole is now almost entirely transferred from the Cu 3d–O 2p state to the Ir 5d–O 2p hybridized orbital. The obtained valences for the B-sites are also listed in Table 1.
Density functional theory (DFT) results
Calculating the spin-polarized electronic structure of the three compounds, as a common feature, we find that the Cu d, B d and O p states are admixed, although the degree of admixture varies between the three compounds. Site-projected partial density of states is shown in Supplementary Fig. 3 (ref. 39). A direct inspection of the plot for CCCoO reveals that the  states, which are strongly admixed with
states, which are strongly admixed with  states, are empty in both spin-up and spin-down channels, thus lending strong support for the
states, are empty in both spin-up and spin-down channels, thus lending strong support for the  valence in CCCoO. This yields a mixed valence of 3.25+ on Co and the intermediate spin state with a magnetic moment of ~1.68 μB. Moving towards the CCRhO compound, the admixture between the
valence in CCCoO. This yields a mixed valence of 3.25+ on Co and the intermediate spin state with a magnetic moment of ~1.68 μB. Moving towards the CCRhO compound, the admixture between the  state and Rh states becomes markedly reduced compared with that of CCCoO. In this compound, the calculated Cu valence is found to be mixed between 2+ and 3+ (~2.5+), with Rh valence close to 3.6+. Unlike Co, magnetically Rh is found to be in the low spin state with a spin moment of 0.21 μB and largely quenched orbital moment of 0.05 μB. Finally, we consider the CCIrO. In sharp contrast to both Co and Rh, the mixing of the
state and Rh states becomes markedly reduced compared with that of CCCoO. In this compound, the calculated Cu valence is found to be mixed between 2+ and 3+ (~2.5+), with Rh valence close to 3.6+. Unlike Co, magnetically Rh is found to be in the low spin state with a spin moment of 0.21 μB and largely quenched orbital moment of 0.05 μB. Finally, we consider the CCIrO. In sharp contrast to both Co and Rh, the mixing of the  p states and the Ir d states is drastically reduced. This results in an almost pure
p states and the Ir d states is drastically reduced. This results in an almost pure  p state occupied in one spin channel and empty in another, implying a Cu2+ valence and nominal Ir4+ valence. The spin moments at Ir and Cu sites are found to be 0.45 μB and 0.65 μB, respectively, with a rather large spin moment of 0.12 μB on O, arising from strong hybridization with Ir. We note that ionic Ir4+ is in the 5d5 configuration and has extensively been discussed in view of the interplay of strong SOC and correlation physics9,10,11,12,13,14,15. The large branching ratio of close to 3.8 signifies the presence of a large SOC at the Ir site, which is found to be common among many of the compounds of Ir4+ in an octahedral environment, even when the compound is metallic as in IrO2 (ref. 38). The orbital moment at the Ir site, calculated within the generalized gradient approximation (GGA)+U+SO, turned out to be 0.12 μB, smaller than the spin moment with morbital/mspin being 0.27. This is curious when compared with the values obtained in cases like BaIrO3 (ref. 11). The structure and coupling mechanisms are, however, rather different between compounds like Sr2IrO4 or BaIrO3 and the present one. In the former examples, magnetic interactions are one- or two-dimensional Ir–Ir, whereas here they are Cu–Ir with three-dimensional connectivity. The dominance of hybridization produces an additional induced spin moment at the Ir site because of the presence of the magnetic ion Cu, a behaviour qualitatively similar to the case of La2CoIrO6, discussed in recent literature37. The presence of a significant SO interaction is found to mix up and down spins and destroys half-metallicity in CCIrO. We note here that inclusion of SOC allows for the non-collinear arrangement of spins. We have therefore carried out spin-polarized calculations assuming collinear as well as non-collinear spin arrangements. The calculated magnetic moments at various atomic sites, obtained in non-collinear calculations, turn out to be very similar to that obtained assuming simple collinear arrangement of spins. The spin magnetic moments are found to be similar to that obtained from collinear calculations within 1–2%, whereas the orbital magnetic moments are found to differ from that in collinear calculation by a maximum of 0.5%, providing confidence in the general conclusion drawn from the electronic structure calculations on the various valence and spin states, irrespective of the assumed spin arrangements.
p state occupied in one spin channel and empty in another, implying a Cu2+ valence and nominal Ir4+ valence. The spin moments at Ir and Cu sites are found to be 0.45 μB and 0.65 μB, respectively, with a rather large spin moment of 0.12 μB on O, arising from strong hybridization with Ir. We note that ionic Ir4+ is in the 5d5 configuration and has extensively been discussed in view of the interplay of strong SOC and correlation physics9,10,11,12,13,14,15. The large branching ratio of close to 3.8 signifies the presence of a large SOC at the Ir site, which is found to be common among many of the compounds of Ir4+ in an octahedral environment, even when the compound is metallic as in IrO2 (ref. 38). The orbital moment at the Ir site, calculated within the generalized gradient approximation (GGA)+U+SO, turned out to be 0.12 μB, smaller than the spin moment with morbital/mspin being 0.27. This is curious when compared with the values obtained in cases like BaIrO3 (ref. 11). The structure and coupling mechanisms are, however, rather different between compounds like Sr2IrO4 or BaIrO3 and the present one. In the former examples, magnetic interactions are one- or two-dimensional Ir–Ir, whereas here they are Cu–Ir with three-dimensional connectivity. The dominance of hybridization produces an additional induced spin moment at the Ir site because of the presence of the magnetic ion Cu, a behaviour qualitatively similar to the case of La2CoIrO6, discussed in recent literature37. The presence of a significant SO interaction is found to mix up and down spins and destroys half-metallicity in CCIrO. We note here that inclusion of SOC allows for the non-collinear arrangement of spins. We have therefore carried out spin-polarized calculations assuming collinear as well as non-collinear spin arrangements. The calculated magnetic moments at various atomic sites, obtained in non-collinear calculations, turn out to be very similar to that obtained assuming simple collinear arrangement of spins. The spin magnetic moments are found to be similar to that obtained from collinear calculations within 1–2%, whereas the orbital magnetic moments are found to differ from that in collinear calculation by a maximum of 0.5%, providing confidence in the general conclusion drawn from the electronic structure calculations on the various valence and spin states, irrespective of the assumed spin arrangements.
The evolution of the nominal valence of Cu from predominant 3+ (d9L) to 2+, as one moves from 3d (Co) to 4d (Rh) to 5d (Ir) elements at the B-site, is controlled by mixing between B-site d states and  p states and can be vividly visualized in the effective Wannier function plots shown in Fig. 4a (upper panel). As clearly seen, the Wannier functions centred at the Cu site have the orbital character of
p states and can be vividly visualized in the effective Wannier function plots shown in Fig. 4a (upper panel). As clearly seen, the Wannier functions centred at the Cu site have the orbital character of  symmetry, and the tail is shaped according to the symmetry of the orbitals mixed with it. Specifically, moving from CCCoO to CCRhO to CCIrO, we find the weight at the tails centred at the B-site (marked by a circle) progressively diminishes.
symmetry, and the tail is shaped according to the symmetry of the orbitals mixed with it. Specifically, moving from CCCoO to CCRhO to CCIrO, we find the weight at the tails centred at the B-site (marked by a circle) progressively diminishes.
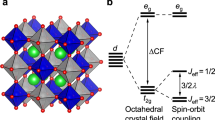
(a) Top panels: plots of effective Wannier functions for O p,  orbitals for CCCoO, CCRhO and CCIrO. Plotted are the constant value surfaces with lobes of different signs coloured as cyan and magenta. The Cu, B- and O-sites are shown as green, red and blue coloued balls. Bottom panels: energy level positions of Cu d, B d and O p states for CCCoO, CCRhO and CCIrO. (b) Doniach phase diagram showing the dependency on the Cu occupation.
orbitals for CCCoO, CCRhO and CCIrO. Plotted are the constant value surfaces with lobes of different signs coloured as cyan and magenta. The Cu, B- and O-sites are shown as green, red and blue coloued balls. Bottom panels: energy level positions of Cu d, B d and O p states for CCCoO, CCRhO and CCIrO. (b) Doniach phase diagram showing the dependency on the Cu occupation.
Microscopically, the nature of this peculiar unmixing/dehybridization effect between Cu–O and B-site in moving from 3d to 4d to 5d element at the B-site can be further elucidated by considering the energy level positions of B d, Cu d and O p states (see Fig. 4a (bottom panel)). As mentioned above, the octahedral crystal field coupled with the trigonal distortion separates the B d states into doubly degenerate  ,
,  and singly degenerate a1g ones, whereas the square planar geometry of the CuO2 plane breaks the Cu d states into
and singly degenerate a1g ones, whereas the square planar geometry of the CuO2 plane breaks the Cu d states into  and the rest, with
and the rest, with  being of the highest energy. In progressing from CCCoO to CCRhO to CCIrO, the relative position of
being of the highest energy. In progressing from CCCoO to CCRhO to CCIrO, the relative position of  with respect to O p states increases, driven by the pushing down of O p states because of the increased crystal field splitting (
with respect to O p states increases, driven by the pushing down of O p states because of the increased crystal field splitting ( splitting) at the B-site. This, in turn, makes the hybridization between the Cu sublattice and B sublattice weaker and weaker as those ions communicate via the intervening oxygen. This highlights a key difference between CCIrO and CaCu3Ru4O12, where for the latter it was found that the suspected Kondo-like physics was unlikely due to a strong mixing of Cu with O40,41. Similar to that of high-Tc cuprates, for CCCoO, the O p states are positioned above
splitting) at the B-site. This, in turn, makes the hybridization between the Cu sublattice and B sublattice weaker and weaker as those ions communicate via the intervening oxygen. This highlights a key difference between CCIrO and CaCu3Ru4O12, where for the latter it was found that the suspected Kondo-like physics was unlikely due to a strong mixing of Cu with O40,41. Similar to that of high-Tc cuprates, for CCCoO, the O p states are positioned above  , placing Cu in to a negative charge transfer regime, which promotes a high-Tc cuprate-like
, placing Cu in to a negative charge transfer regime, which promotes a high-Tc cuprate-like  state akin to the Zhang-Rice singlet state24,25,26,42. The progressive weakening of covalency between the B sublattice and Cu–O sublattice, as one moves from CCCoO to CCRhO to CCIrO, makes the spread of the effective
state akin to the Zhang-Rice singlet state24,25,26,42. The progressive weakening of covalency between the B sublattice and Cu–O sublattice, as one moves from CCCoO to CCRhO to CCIrO, makes the spread of the effective  Wannier function (top panel of Fig. 4a) in the case of Ir dramatically reduced compared with either Co or Rh.
Wannier function (top panel of Fig. 4a) in the case of Ir dramatically reduced compared with either Co or Rh.
Unified picture
Finally, the element-resolved spectroscopic results combined with the ab-initio calculations prompts us to build a unified framework to explain their emergent physical behaviour. Although an earlier study utilizing electron energy loss spectroscopy found small changes between 3–4–5 d A-site-ordered perovskites from different columns23, our study reveals that upon ascending a column of the periodic table from Ir to Co, the  orbital occupation changes from practically ionic
orbital occupation changes from practically ionic  for CCIrO to the non-magnetic cuprate Zhang-Rice-like state with
for CCIrO to the non-magnetic cuprate Zhang-Rice-like state with  for CCCoO, as supported by experimentally and theoretically deduced valences listed in Table 1. Along with it, these localized and magnetically active Cu d states in CCIrO shift towards the Fermi surface demonstrating a rapid change in hybridization compared with both CCRhO and CCCoO. On the opposite end, such a drastic change in the Cu orbital occupation results in the mixed valence intermediate spin state of Co3.25+, mixed valence Rh~3.7+ but ionic Ir4+ (5d5). These findings allow us to place the three compounds under discussion in the context of the Doniach phase diagram depicted in Fig. 4b, where the fundamental control parameter is the average occupation ‹n›Cu of the
for CCCoO, as supported by experimentally and theoretically deduced valences listed in Table 1. Along with it, these localized and magnetically active Cu d states in CCIrO shift towards the Fermi surface demonstrating a rapid change in hybridization compared with both CCRhO and CCCoO. On the opposite end, such a drastic change in the Cu orbital occupation results in the mixed valence intermediate spin state of Co3.25+, mixed valence Rh~3.7+ but ionic Ir4+ (5d5). These findings allow us to place the three compounds under discussion in the context of the Doniach phase diagram depicted in Fig. 4b, where the fundamental control parameter is the average occupation ‹n›Cu of the  orbital modulated by the hybridization from the strongly mixed B-site d- and O p-bands43,44,45. In the modern version of the Doniach phase diagram, interesting physics involving heavy fermions manifests itself as a competition between the Kondo liquid and spin liquid behaviour mediated by chemical doping, whereas very little attention has been given to the mixed valency regime, particularly in d-electron systems and in the absence of doping43,44,45,46,47,48. In this framework, the overall ground state is defined by the competition between RKKY-type magnetic exchange between magnetic holes on Cu with the Kondo screening by conduction carries from the B–O sublattices. For CCIrO, with a S=1/2 d-hole localized on Cu, the large magnetic exchange is comparable in strength with the Kondo screening, resulting in the strongly enhanced effective mass observed with transport and thermal measurements27. Thus, Cheng et al. firmly placed CCIrO into the heavy fermion regime I in Fig. 4b with the antiferromagnetic local moment short-range magnetism23,27. In moving from Ir to Rh and Co, the Kondo energy scale begins to gain because of the collective hybridization of Cu d-holes into the ZR singlets. With the strong reduction in the
orbital modulated by the hybridization from the strongly mixed B-site d- and O p-bands43,44,45. In the modern version of the Doniach phase diagram, interesting physics involving heavy fermions manifests itself as a competition between the Kondo liquid and spin liquid behaviour mediated by chemical doping, whereas very little attention has been given to the mixed valency regime, particularly in d-electron systems and in the absence of doping43,44,45,46,47,48. In this framework, the overall ground state is defined by the competition between RKKY-type magnetic exchange between magnetic holes on Cu with the Kondo screening by conduction carries from the B–O sublattices. For CCIrO, with a S=1/2 d-hole localized on Cu, the large magnetic exchange is comparable in strength with the Kondo screening, resulting in the strongly enhanced effective mass observed with transport and thermal measurements27. Thus, Cheng et al. firmly placed CCIrO into the heavy fermion regime I in Fig. 4b with the antiferromagnetic local moment short-range magnetism23,27. In moving from Ir to Rh and Co, the Kondo energy scale begins to gain because of the collective hybridization of Cu d-holes into the ZR singlets. With the strong reduction in the  orbital occupation, both CCRhO and CCCoO enter the regime II of mixed valency (or Kondo liquid phase) in Fig. 4b. Unlike regime I, in the mixed valence regime quantum fluctuations between different electronic configurations are highly relevant; in this regime, the local electronic and magnetic structure of Kondo centres (Cu) is defined by the redistribution of electrons between Cu d states and electrons from the strongly hybridized d- and p-states of Rh (Co) and O, that is, |3d9, S=1/2› versus
orbital occupation, both CCRhO and CCCoO enter the regime II of mixed valency (or Kondo liquid phase) in Fig. 4b. Unlike regime I, in the mixed valence regime quantum fluctuations between different electronic configurations are highly relevant; in this regime, the local electronic and magnetic structure of Kondo centres (Cu) is defined by the redistribution of electrons between Cu d states and electrons from the strongly hybridized d- and p-states of Rh (Co) and O, that is, |3d9, S=1/2› versus  . The conjectured microscopic framework that links the electronic and magnetic ground state of the A-site perovskites with macroscopic behaviour opens a path in designing emergent-ordered phases with heavy fermion behaviour, quantum criticality and unconventional superconductivity in the magnetic Kondo lattice of cuprate-like moments.
. The conjectured microscopic framework that links the electronic and magnetic ground state of the A-site perovskites with macroscopic behaviour opens a path in designing emergent-ordered phases with heavy fermion behaviour, quantum criticality and unconventional superconductivity in the magnetic Kondo lattice of cuprate-like moments.
To summarize, we performed XAS measurements and first-principles calculations on a series of A-site-ordered perovskites, chemical formula CaCu3B4O12, spanning one period of the periodic table. Surprisingly, we find that the materials fit well within the Doniach phase diagram, being controlled by the hole count on Cu, leading to the conclusion that the competition between RKKY and Kondo effects is responsible for the anomalous behaviour observed in the CCIrO compound.
Methods
Sample preparation
All samples used in the present study were prepared under high-pressure and high-temperature conditions with a Walker-type multianvil module (Rockland Research Co.). The A-site-ordered perovskites CCCoO, CCRhO and CCIrO were obtained under P=9 GPa and T=1,000–1,300 °C; the reference perovskites SrRhO3 and SrIrO3 were obtained at 8 GPa, 1,200 °C and 6 GPa, 1,000 °C, respectively. About 30 wt.% KClO4 acting as oxidizing agent were added for synthesizing the compounds containing Co and Rh. The resultant KCl was washed away with deionized water. Phase purity of the above samples was examined with powder X-ray diffraction at room temperature with a Philips Xpert diffractometer (Cu Kα radiation). All the A-site-ordered perovskites adopt a cubic  structure with lattice parameter increasing progressively from a=7.1259(3) Å for M=Co to a=7.3933(1) Å for M=Rh, and to a=7.4738(1) Å for M=Ir. On the other hand, the reference perovskites crystallize into the orthorhombic Pbnm structure with unit-cell parameters a=5.5673(1) Å, b=5.5399(2) Å and c=7.8550(2) Å for SrRhO3, and a=5.5979(1) Å, b=5.5669(1) Å and c=7.8909(1) Å for SrIrO3, respectively.
structure with lattice parameter increasing progressively from a=7.1259(3) Å for M=Co to a=7.3933(1) Å for M=Rh, and to a=7.4738(1) Å for M=Ir. On the other hand, the reference perovskites crystallize into the orthorhombic Pbnm structure with unit-cell parameters a=5.5673(1) Å, b=5.5399(2) Å and c=7.8550(2) Å for SrRhO3, and a=5.5979(1) Å, b=5.5669(1) Å and c=7.8909(1) Å for SrIrO3, respectively.
X-ray measurements
XAS measurements were carried out on the polycrystalline samples in the soft X-ray branch at the 4-ID-C beamline in the bulk-sensitive TFY and total electron yield modes, with a 0.1 eV (0.3 eV) resolution at the O K-edge (Cu L-edge), at the Advanced Photon Source in Argonne National Laboratory. Measurements were taken on the Cu L-edge and O K-edge for all samples, and all measurements shown here were obtained in total electron yield mode (TFY available in Supplementary Fig. 1). To measure the 4d and 5d B-site valences, hard XAS measurements with a 1.5(3) eV resolution were taken at the 4-ID-D beamline in transmission (fluorescence) mode for Ir (Rh).
Computational details
In the first-principles DFT calculations, we have primarily used the plane wave basis set and pseudo-potentials as implemented in the Vienna Ab-initio Simulation Package49. The exchange-correlation function was chosen to be that of the GGA implemented following the parametrization of Perdew-Burke-Ernzerhof50. The electron–electron correlation beyond GGA was taken into account through improved treatment of GGA+U calculation within the +U implementation of Dudarev et al.51 For the plane wave-based calculations, we used projector augmented wave52 potentials. The wave functions were expanded in the plane wave basis with a kinetic energy cutoff of 600 eV and Brillouin zone (BZ) summations were carried out with a 6 × 6 × 6 k-mesh. A U-value of 5 eV on Cu site was used. For the U-value on the B site, a value of 4 eV was used for the 3d element Co and 1–2 eV was used for 4d and 5d elements, Rh and Ir. The obtained results were verified in terms of variation of U parameter. The Hund’s rule coupling J was fixed to 0.8 eV. The plane wave results were verified in terms of full-potential linearized augmented plane wave method as implemented53 in WIEN2k. For FLAPW calculations, we chose the augmented plane wave+lo as the basis set and the expansion in spherical harmonics for the radial wave functions was taken up to l=10. The charge densities and potentials were represented by spherical harmonics up to l=6. For BZ integration, we considered about 200 k-points in the irreducible BZ and modified tetrahedron method was applied54. The commonly used criterion for the convergence of basis sets relating the plane wave cutoff, Kmax, and the smallest atomic sphere radius, RMT, RMT*Kmax, was chosen to be 7.0. Spin–orbit coupling has been included in the calculations in scalar relativistic form as a perturbation to the original Hamiltonian.
To estimate the positions of the Cu d, B d and O p energy levels as well as the plots of the effective Wannier functions for B d states, we used muffin-tin orbital (MTO)-based N-th order MTO (NMTO)55-downfolding calculations. Starting from full DFT calculations, NMTO-downfolding arrives at a few-orbital Hamiltonian by integrating out degrees, which are not of interest. It does so by defining energy-selected, effective orbitals, which serve as Wannier-like orbitals defining the few-orbital Hamiltonian in the downfolded representation. NMTO technique, which is not yet available in its self-consistent form, relies on the self-consistent potential parameters obtained out of linear MTO56 calculations. The results were cross-checked among the calculations in three different basis sets in terms of total energy differences, density of states and band structures.
Additional information
How to cite this article: Meyers, D. et al. Competition between heavy fermion and Kondo interaction in isoelectronic A-site-ordered perovskites. Nat. Commun. 5:5818 doi: 10.1038/ncomms6818 (2014).
References
Imada, M., Fujimori, A. & Tokura, Y. Metal-insulator transitions. Rev. Mod. Phys. 70, 1039–1263 (1998).
Chakhalian, J. et al. Magnetism at the interface between ferromagnetic and superconducting oxides. Nat. Phys. 2, 244–248 (2006).
Chakhalian, J. et al. Orbital Reconstruction and covalent bonding at an oxide interface. Science 318, 1114–1117 (2007).
Tokura, Y. Critical features of colossal magnetoresistive manganites. Rep. Prog. Phys. 69, 797–851 (2006).
McCormack, M., Jin, S., Tiefel, T. H., Fleming, R. M. & Phillips, J. M. Very large magnetoresistance in perovskite-like La-Ca-Mn-O thin films. Appl. Phys. Lett. 64, 3045–3047 (1994).
Wang, J. et al. Epitaxial BiFeO3 multiferroic thin film heterostructures. Science 299, 1719–1722 (2003).
Zaanen, J., Sawatzky, G. A. & Allen, J. W. Band gaps and electronic structure of transition-metal compounds. Phys. Rev. Lett. 55, 418–421 (1985).
Tokura, Y. & Nagaosa, N. Multiferroics as quantum electromagnets. Science 69, 1481–1482 (2006).
Haskel, D. et al. Pressure tuning of the spin-orbit coupled ground state in Sr2IrO4 . Phys. Rev. Lett. 109, 027204 (2012).
Kim, B. J. et al. Novel Jeff=1/2 Mott state induced by relativistic spin-orbit coupling in Sr2IrO4 . Phys. Rev. Lett. 101, 076402 (2008).
Laguna-Marco, M. A. et al. Orbital magnetism and spin-orbit effects in the electronic structure of BaIrO3 . Phys. Rev. Lett. 105, 216407 (2010).
Witczak-Krempa, W., Chen, G., Kim, Y. B. & Balents, L. Correlated quantum phenomena in the strong spin-orbit regime. Annu. Rev. Condens. Mater. Phys 5, 57–82 (2014).
Cao, G., Bolivar, J., McCall, S., Crow, J. E. & Guertin, R. P. Weak ferromagnetism, metal-to-nonmetal transition, and negative differential resistivity in single-crystal Sr2IrO4 . Phys. Rev. B 57, 11039(R) (1998).
Cao, G. et al. Anomalous magnetic and transport behavior in the magnetic insulator Sr3Ir2O7 . Phys. Rev. B 66, 214412 (2002).
Ashcroft, N. W. & Mermin, N. D. Solid State Physics, Dorothy Garbose Crane 659Saunders College Publishing (1976).
McGuinness, C. et al. X-ray spectroscopic study of the electronic structure of the high-dielectric-constant material CaCu3Ti4O12 . Phys. Rev. B 71, 19511 (2005).
Shiraki, H. et al. Ferromagnetic cuprates CaCu3Ge4O12 and CaCu3Sn4O12 with A-site ordered perovskite structure. Phys. Rev. B 76, 140403(R) (2007).
Yamada, I. et al. A perovskite containing quadrivalent iron as a charge-disproportionated ferrimagnet. Angew. Chem. Int. Ed. 47, 7032–7035 (2008).
Morita, Y. et al. Valence fluctuations and correlated metallic states in A-site ordered perovskite oxides ACu3V4O12 (A=Na, Ca, and Y). Phys. Rev. B 81, 165111 (2010).
Hollmann, N. et al. Correlation effects in CaCu3Ru4O12 . Phys. Rev. B 87, 155122 (2013).
Chen, W.-T. et al. Ligand-hole localization in oxides with unusual valence Fe. Sci. Rep 2, 449 (2012).
Yamada, I. et al. Synthesis, structure, and physical properties of A-site ordered perovskites ACu3Co4O12 (A=Ca and Y). Chem. Mater. 22, 5328–5332 (2010).
Xin, Y. et al. Study of atomic structure and electronic structure of an AA′3B4O12 double-perovskite CaCu3Ir4O12 using STEM imaging and EELS techniques. Ultramicroscopy 127, 94–99 (2013).
Meyers, D. et al. Zhang-Rice physics and anomalous copper states in A-site ordered perovskites. Sci. Rep. 3, 1834 (2013).
Mizokawa, T. et al. Metallic versus insulating behavior in the A-site ordered perovskite oxides ACu3Co4O12 (A=Ca and Y) controlled by Mott and Zhang-Rice physics. Phys. Rev. B 80, 125105 (2009).
Zhang, F. C. & Rice, T. M. Effective hamiltonian for the superconducting Cu oxides. Phys. Rev. B 37, 3759–3761 (1988).
Cheng, J.-G. et al. Possible Kondo Physics near a metal-insulator crossover in the A-Site ordered perovskite CaCu3Ir4O12 . Phys. Rev. Lett. 111, 176403 (2013).
Achkar, A. J. et al. Bulk sensitive X-ray absorption spectroscopy free of self-absorption effects. Phys. Rev. B 83, 081106(R) (2011).
Sarangi, R. et al. X-ray absorption edge spectroscopy and computational studies on LCuO2 species. J. Am. Chem. Soc. 128, 8286–8296 (2006).
Hu, Z. et al. On the electronic structure of Cu(III) and Ni(III) in La2Li1/2Cu1/2O4, Nd2Li1/2Ni1/2O4, and Cs2KCuF6 . Chem. Phys. 232, 63–74 (1998).
Kaindl, G. et al. Correlation between oxygen-hole concentration and Tc in YBa2Cu3O7−δ from Cu-LIII X-ray absorption. Phys. B Condens. Matter 158, 446–449 (1989).
Chen, C. T. Electronic states in La2−xSrxCuO4+δ probed by soft-X-Ray absorption. Phys. Rev. Lett. 66, 104–107 (1991).
Nücker, N., Fink, J., Fuggle, J. C., Durham, P. J. & Temmerman, W. M. Evidence for holes on oxygen sites in the high Tc superconductors La2−xSrxCuO4 and YBa2Cu3O7−γ . Phys. Rev. B 37, 5158–5163 (1988).
Nücker, N. et al. Site-specific and doping-dependent electronic structure of YBa2Cu3Ox probed by O 1s and Cu 2p X-ray-absorption spectroscopy. Phys. Rev. B 51, 8529–8542 (1995).
Kuiper, P. et al. X-ray absorption study of the O 2p hole concentration dependence on O stoichiometry in YBa2Cu3Ox . Phys. Rev. B 38, 6483–6489 (1988).
Zeng, Z., Greenblatt, M., Subramanian, M. A. & Croft, M. Large Low-Field Magnetoresistance in perovskite-type CaCu3Mn4O12 without double exchange. Phys. Rev. Lett. 82, 3164–3167 (1999).
Kolchinskaya, A. et al. Magnetism and spin-orbit coupling in Ir-based double perovskites La2−xSrxCoIrO6 . Phys. Rev. B 85, 224422 (2012).
Clancy, J. P. et al. Spin-orbit coupling in iridium-based 5d compounds probed by X-ray absorption spectroscopy. Phys. Rev. B 86, 195131 (2012).
Mukherjee, S., Sarkar, S. & Saha-Dasgupta, T. First principles study of CaCu3B4O12 (B=Co, Rh, Ir). J. Mater. Sci. 47, 7660–7664 (2012).
Mizumaki, M. et al. Oxygen hole state in A-site ordered perovskite ACu3Ru4O12 (A=Na, Ca, and La) probed by resonant X-ray emission spectroscopy. J. Phys. Soc. Jpn 82, 024709 (2013).
Tanaka, S., Takatsu, H., Yonezawa, S. & Maeno, Y. Suppression of the mass enhancement in CaCu3Ru4O12 . Phys. Rev. B 80, 035113 (2009).
Ushakov, A. V., Streltsov, S. V. & Khomskii, D. I. Crystal field splitting in correlated systems with negative charge-transfer gap. J. Phys. Condens. Matter 23, 445601 (2011).
Yang, Y.-I. & Pines, D. Emergent states in heavy-electron materials. Proc. Natl Acad. Sci. USA 109, 3060–3066 (2012).
Süllow, S., Aronson, M. C., Rainford, B. D. & Haen, P. Doniach Phase diagram, revisited: from ferromagnet to fermi liquid in pressurized CeRu2Ge2 . Phys. Rev. Lett. 82, 2963–2966 (1999).
Si, Q. Global magnetic phase diagram and local quantum criticality in heavy fermion metals. Phys. B Condens. Matter 378, 23–27 (2006).
Yoshida, K. Magnetic properties of Cu-Mn alloys. Phys. Rev 106, 893–898 (1957).
Pandey, A., Mazumdar, C., Ranganathan, R. & Dattagupta, S. Magnetism in ordered metallic perovskite compound GdPd3BxC1−x . J. Mag. Mag. Mat. 321, 2311–2317 (2009).
Feng, J., Xiao, B., Zhou, R. & Pan, W. Electronic and magnetic properties of double perovskite slab-rocksalt layer rare earth strontium aluminates natural superlattice structure. J. Apl. Phys. 114, 143907 (2013).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Dudarev, S. L. et al. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 57, 1505–1509 (1998).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Blaha, P., Schwartz, K., Madsen, G. K. H., Kvasnicka, D. & Luitz, J. inAn Augmented Plane Wave+Local Orbitals Program for Calculating Crystal Properties ed. Schwarz K. Technische Universitatâ Wien (2001).
Blöchl, P. E., Jepsen, O. & Andersen, O. K. Improved tetrahedron method for Brillouin-zone integrations. Phys. Rev B 49, 16223 (2004).
Andersen, O. K. & Saha-Dasgupta, T. Muffin-tin orbitals of arbitrary order. Phys. Rev. B 62, R16219 (2000).
Andersen, O. K. & Jepsen, O. Explicit, first-principles tight-binding theory. Phys. Rev. Lett. 53, 2571–2574 (1984).
Acknowledgements
J.C. is supported by DOD-ARO Grant No. 0402-17291. J.-S.Z. and J.B.G. are supported by NSF Grant. No. DMR-1122603. T.S.-D. thank CSIR and DST, India, for funding. Work at the Advanced Photon Source, Argonne, is supported by the US Department of Energy, Office of Science under Grant No. DEAC02-06CH11357. J.-G.C. acknowledges the support from NSFC and MOST (Grant Nos. 11304371, 2014CB921500) and the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB07000000). We thank Dr Shalinee Chikara and Professor Gang Cao for sharing data on reference samples Rh2O3 and Sr2RhO4. J.C. acknowledges useful discussions with D. Khomskii.
Author information
Authors and Affiliations
Contributions
D.M., S. Middey, Y. Choi, D.H., B.A.G., J.W.F. and J.C. acquired the experimental data. S. Mukherjee and T.S.-D. did the theoretical calculations. J.-G.C., J.-S.Z. and J.B.G. grew the samples. D.M. and S.Middey analysed data. All authors discussed the results. D.M., S. Middey, Y. Cao., T.S.-D. and J.C. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-3 and Supplementary References. (PDF 801 kb)
Rights and permissions
About this article
Cite this article
Meyers, D., Middey, S., Cheng, JG. et al. Competition between heavy fermion and Kondo interaction in isoelectronic A-site-ordered perovskites. Nat Commun 5, 5818 (2014). https://doi.org/10.1038/ncomms6818
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms6818
This article is cited by
-
Ligand size effects in two-dimensional hybrid copper halide perovskites crystals
Communications Materials (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.