Abstract
The sources of dissolved inorganic carbon (DIC) used to produce scleractinian coral skeletons are not understood. Yet this knowledge is essential for understanding coral biomineralization and assessing the potential impacts of ocean acidification on coral reefs. Here we use skeletal boron geochemistry to reconstruct the DIC chemistry of the fluid used for coral calcification. We show that corals concentrate DIC at the calcification site substantially above seawater values and that bicarbonate contributes a significant amount of the DIC pool used to build the skeleton. Corals actively increase the pH of the calcification fluid, decreasing the proportion of DIC present as CO2 and creating a diffusion gradient favouring the transport of molecular CO2 from the overlying coral tissue into the calcification site. Coupling the increases in calcification fluid pH and [DIC] yields high calcification fluid [CO32−] and induces high aragonite saturation states, favourable to the precipitation of the skeleton.
Similar content being viewed by others
Introduction
Neither the sources1 nor the species2,3 of dissolved inorganic carbon (DIC) used during coral calcification are understood. The aragonite skeleton precipitates from an extracellular calcification fluid (ECF) enclosed in a semi-isolated space between the skeleton and the overlying coral tissue. The DIC utilized to form the coral skeleton is derived from seawater and from an internal DIC pool1,4. An active bicarbonate transporter has not been ruled out in coral, but dual-radiolabelling studies suggest that this is not the source of additional carbon4. The isotopically light carbon and oxygen compositions of coral skeletons suggest that molecular CO2 may act as the source of internal DIC5. Understanding the sources of skeletal carbon is key to the accurate prediction of the effects of increasing [DIC] in seawater (ocean acidification) and for the correct interpretation of δ18O and δ13C coral-based palaeoenvironmental records.
We analysed the B/Ca and B isotope ratios (δ11B) of coral aragonite to reconstruct the DIC chemistry of the coral ECF. Dissolved boron in seawater occurs primarily as boric acid, B(OH)3, and borate, B(OH)4−, and speciation is controlled by ambient pH6. Borate is selectively incorporated into aragonite7, presumably substituting for CO32− in the lattice. There are no known active transport mechanisms for boron in corals and we assume that dissolved boron is transported to the ECF in seawater. Seawater transport to the ECF is a passive process8 and as such the transport rate is likely to be constant. At equilibrium, B(OH)3 is enriched in 11B compared with B(OH)4− (ref. 9), hence the δ11B of coral aragonite reflects ECF pH. Skeletal [B] reflects both ECF pH and the concentration of the DIC species competing with borate for inclusion in the carbonate10.
Passive diffusion of B(OH)3 across cell membranes11 could potentially offset ECF δ11B from seawater values. However, ECF pH estimates derived from skeletal δ11B compare well with direct characterizations using fluorescent probes, suggesting that this effect is insignificant, that is, the observed ECF pH in Stylophora pistillata (8.69 and 8.36 in the light and dark, respectively) cultured at seawater pH 8.1 (ref. 12) is in excellent agreement with δ11B of the same species cultured at pH 8.09 (24.8‰, equivalent to an ECF pH of 8.55)13, assuming that calcification is three times faster in the light than in the dark14. A recent suggestion that skeletal δ11B ECF pH estimates may be offset to lower values than expected15 is based on a comparison of skeletal δ11B and direct ECF pH measurements in the light only. ECF pH is lower in the dark16 and this likely accounts for the offset.
We used skeletal δ11B to estimate ECF pH17 and B/Ca to estimate the concentration of the DIC species that co-precipitates with B(OH)4−. It is not clear whether CO32− or HCO3− ions are utilized during coral aragonite precipitation2,3. We consider three scenarios: that B(OH)4− co-precipitates with CO32−only (scenario 1), with HCO3− only (scenario 2) or with both CO32− and HCO3− (scenario 3). We estimated the B(OH)4−/CO32−, B(OH)4−/HCO3− and B(OH)4−/(CO32−+HCO3−) aragonite partition coefficients from an estimate of the δ11B and B/Ca of secondary aragonite cement in a fossil coral coupled with alkalinity measurements of coral skeletal pore fluids18. We used our estimates of ECF pH and co-precipitating DIC species to calculate the concentrations of the other carbonate system variables in the ECF, namely, all other DIC species and total alkalinity (TA). We show that the ECF pH and DIC chemistry are significantly different from that of seawater and that bicarbonate contributes a significant amount of the DIC pool used to build the skeleton.
Results and Discussion
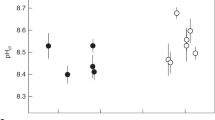
Modern Porites spp. field corals
We analysed three modern massive Porites spp. field corals from Oahu, Hawaii, and Jarvis Island, South Pacific. Skeletal δ11B ECF pH estimates (Fig. 1; Table 1) confirm that corals actively increase the pH of the ECF above that of seawater12,16. The ECF composition reflects the balance of DIC inputs and outputs, namely, seawater diffusion, molecular CO2 invasion, proton extrusion and calcification (Fig. 2). CO2 invasion does not influence TA and the departure of ECF [TA] from seawater values reflects calcification (reduces [TA]) and proton extrusion (increases [TA]). Similarly, proton extrusion does not affect [DIC] and the departure of ECF [DIC] from seawater values reflects calcification (reduces [DIC]) and CO2 invasion (increases [DIC]).
(a) ECF pH (from skeletal δ11B), (b) skeletal B/Ca and (c) reconstructed ECF DIC system parameters. Ω, aragonite saturation state. Both δ11B and B/Ca are normally distributed in each coral and error bars are 95% confidence limits (s.e.m.). Data are means of ≥99 B/Ca analyses and ≥40 δ11B analyses with the exception of δ11B in the Jarvis coral where only 12 analyses were made, note the larger confidence limits. DIC system errors are calculated from propagating 95% confidence limits in B/Ca and δ11B analyses onto DIC system estimates. Horizontal lines denote seawater concentrations and are calculated from observations of pH and TA in Jarvis Island benthic reefwater29 and DIC and TA in Hawaii reefwater33.
Under scenario 1 (borate co-precipitates with CO32−), the ECF has [DIC] and [TA], which are substantially lower than ambient seawater (Fig. 1). Ca-ATPase activity has little effect on the [Ca] of the ECF19 and in scenario 1, the aragonite saturation state (Ω) of the ECF (essentially a product of [Ca] and [CO32−]) is ~3 and below that of seawater (Ω=~4). It is implausible that the high calcification rates observed in tropical corals are attained with such an impoverished ECF and we reject this scenario.
Scenario 2 (borate co-precipitates with HCO3−) and scenario 3 (borate co-precipitates with both HCO3− and CO32−) produce broadly similar results (Fig. 1), as HCO3− is the most abundant carbon species (70–90%) over the observed ECF pH range. Proton pumping maintains ECF TA in the untreated corals well above seawater concentrations and [DIC] is up to double that of ambient seawater (Fig. 1). ECF Ω in the untreated corals rises to 11–19, depending on coral and scenario, and facilitates rapid aragonite precipitation. These are both credible scenarios. Our observation that borate co-precipitates with HCO3− in the aragonite lattice indicates that a large proportion of skeletal carbon is ultimately derived from the bicarbonate of the ECF. It is unknown whether HCO3− deprotonates before or after binding to the aragonite20, but the key point is that bicarbonate contributes to the DIC pool used during calcification.
Under scenarios 2 and 3, up to half of the DIC used in calcification does not come though the seawater transport pathway. The enzyme-driven increase in ECF pH shifts the DIC equilibrium in favour of CO32− at the expense of CO2 and HCO3−, creating a diffusion gradient favouring the diffusion of molecular CO2 from the overlying coral tissue into the ECF21. We conclude that this is a likely source of the additional skeletal carbon. This interpretation is supported by geochemical and modelling studies, which indicate that a substantial proportion of coral aragonite is derived from an isotopically light (with respect to carbon and oxygen) molecular CO2 source5,21. We note that ECF [CO2] in the field corals is significantly below that of seawater. Either CO2 diffusion rate into the ECF is rate limited or the mean [CO2] in the overlying coral tissue is reduced below that of seawater.
Cultured Pocillopora damicornis
To investigate this further, we analysed cultured colonies of the branching coral, Pocillopora damicornis, some of which were incubated with the Ca-ATPase enzyme inhibitor, ruthenium red22. Ca-ATPase pumps Ca2+ into and H+ out of the ECF and increases ECF pH19. Ruthenium red solutions absorb light from 430 to 615 nm; however, the collective evidence suggests that the chemical reduces coral calcification rate by directly inhibiting Ca-ATPase rather than by inhibiting zooxanthellar photosynthesis22.
This experiment was designed to explore the response of ECF DIC chemistry to changes in ECF pH and all the cultured colonies were grown (by asexual budding and division) from branches of a single-parent colony, that is, all colonies were genetically identical. We do not infer that the ECF DIC chemistry of the cultured colonies is representative of this coral species in the field. We cultured duplicate corals in each treatment and in the solvent control and a single colony in the seawater control. We model the ECF DIC chemistry for each coral colony separately (Fig. 3). Inhibition of Ca-ATPase decreased skeletal δ11B (and ECF pH) in the high ruthenium red treatment compared with both the seawater and the dimethyl sulfoxide (DMSO) controls.
(a) ECF pH (from skeletal δ11B), (b) skeletal B/Ca and (c) reconstructed ECF DIC system parameters. Ω, aragonite saturation state. Error bars are 95% confidence limits (s.e.m.). Cultured corals were exposed to ruthenium red (RR) dissolved in 0.1% DMSO and DMSO and seawater-only controls (con.) were also analysed. Corals were analysed in duplicate (indicated by 1 or 2 annotation). Analyses (7–16) were collected on each sample (Table 1) with the exceptions of RR 3.7 μM 2 and RR 5.3 μM 1 where only 2 and 1, respectively, credible δ11B analysis (exhibiting the 42Ca spike throughout the analysis) were obtained. Errors are calculated as for the field corals and horizontal lines denote seawater concentrations calculated from observations of pH and TA in the culture seawater22.
However, there is little variation in reconstructed ECF [CO2] despite the large ECF pH range observed in these specimens. To illustrate the relationships between ECF pH and DIC chemistry, we plotted ECF [DIC] and Ω as a function of ECF pH and CO2 assuming that CO2 diffuses freely into the ECF maintaining equilibrium with an overlying CO2 source (Fig. 4). ECF DIC increases with increasing pH, reflecting the conversion of CO2 to other DIC species at high pH and the diffusion of additional CO2 into the ECF, thereby concentrating DIC. Ω increases with increasing pH, reflecting the increase in ECF [CO32−] with pH. The data sets from both the cultured and field corals indicate that ECF [CO2] is approximately a third to a half of that in ambient seawater across all treatments. We are unable to determine whether this reflects an equilibrium with the overlying CO2 source or indicates a rate limitation on the diffusion of CO2 into the ECF. We find a strong positive correlation between ECF aragonite saturation state and coral calcification rate (Fig. 5), although we note that the rate dependence in coral is less than that in aragonite inorganically precipitated at the same temperature, that is, doubling aragonite saturation state increases precipitation by 1.5 × in coral and 5 × in synthetic aragonite23. This is perhaps to be expected. Corals do not precipitate randomly but exert exquisite control over both the sites of precipitation and the crystal morphology24.
The relationships were modelled under a range of CO2 scenarios. (a) ECF [DIC] and (b) ECF aragonite saturation state (Ω) increase with ECF pH, assuming that CO2 readily diffuses into the calcification site, maintaining an equilibrium with an overlying [CO2] ranging from that of ambient seawater to 1/8 of this. DIC calculations were made using CO2 sys30, assuming seawater T=25 °C and S=40.6, that is, the conditions in the culture seawater. Reconstructed DIC parameters under scenario 3 in the field and cultured corals are overlain on each graph. Field corals grew under different temperatures and salinities, but this does not affect the interpretation of these graphs. Error bars are 95% confidence limits (s.e.m.) and in the case of ECF DIC are smaller than the heights of the symbols.
Reconstructed calcification fluid aragonite saturation state (Ω) was calculated under scenario 3. Colony calcification rate is calculated from the mean calcification rate measured in the presence of the inhibitor, if used, as a proportion of the calcification rate observed on day 1, before the introduction of the inhibitor22.
Our findings have implications for predicting the effects of ocean acidification on coral reefs. Although the ECF pH gradient facilitates the diffusion of CO2 into the calcification site, we find that ECF [CO2] in all field corals is significantly below that of seawater. Ocean acidification decreases seawater pH but increases seawater [DIC] and [CO2]. Understanding how these changes will impact both ECF pH and the diffusion of CO2 into the coral ECF as a carbon concentration mechanism is key to interpreting current ocean acidification studies and predicting the effects of future scenarios.
Methods
Sample processing
For further details of field sites and coral-culturing procedures, see refs 17, 22, 25, 26. Cultured corals were originally collected from the Gulf of Eilat, and were maintained in seawater at ambient salinity (40.6). Each culture experiment lasted for 5 days. Individual colonies were placed in flow-through coral chambers at the start of day 1 and ruthenium red dissolved in DMSO solution to a final concentration of 0.1% DMSO was added to the seawater supplied to the chambers at the start of day 2. A stable isotope tracer (42Ca as 42CaCO3) was added to the seawater at the same time as the inhibitor, allowing accurate identification of skeleton deposited in the presence of the inhibitor (if used) by secondary ion mass spectrometry (SIMS). This tracer increased the seawater 42Ca/44Ca from 0.31 to ~0.40 and increased the [Ca] of seawater by ~0.2%. A DMSO control (0.1%) and a seawater control (with the addition of 42CaCO3 only) were also tested. All treatments were tested in duplicate. Light and dark coral calcification rates were estimated each day22 using the alkalinity anomaly technique2.
Modern field and cultured corals were living when collected/sacrificed. Specimens were immersed in 3–4% sodium hypochlorate (I) solutions for ≥8 h with intermittent ultrasonic agitation to remove organic contamination, then rinsed repeatedly in distilled water and dried. Skeletal strips were sawn along the maximum growth axis of the field specimens, divided into 10–15 mm lengths and fixed to 25 mm round thin sections. Branch ends of cultured corals were fixed into 25 mm circular epoxy resin blocks (Epofix, Struers). Sections and blocks were polished using silicon carbide papers (up to 4,000 grade, lubricated with water) and polishing alumina (0.05 μm, suspended in water). Multiple SIMS analyses were evenly spaced across 1 year (Jarvis coral) and 2 years (both Hawaiian corals) of skeletal growth in the field corals. Annual growth bands were identified from X-ray radiographs in the Hawaiian corals and from unpublished δ13C data, which exhibit a seasonal trend, in the Jarvis coral. No analyses were made in the outermost parts of the field skeletons, which contained the tissue layers of the corals. In the cultured corals, SIMS analyses were sited on the outermost tips of the skeleton. Analyses that did not exhibit enhanced 42Ca/44Ca throughout the analysis were rejected. Sections were repolished between batches of analyses to expose fresh areas for SIMS.
δ11B and B/Ca analyses
Skeletal δ11B and B/Ca were determined by SIMS in the School of GeoSciences at the University of Edinburgh. The high spatial resolution of SIMS (primary beam diameters=25–40 μm) allows the selective analysis of both the primary coral aragonite, avoiding contamination from secondary cements or microboring organisms, and the small skeletal volumes deposited in the culture experiment. δ11B in the Hawaiian and cultured corals were analysed with a Cameca 1270, while the Jarvis coral was analysed with a Cameca 4f. One coral (Hawaii 1) had also previously been analysed using the Cameca 4f17 and there is excellent agreement in standardized δ11B estimates between the two instruments (within 0.4‰, equivalent to a pH of 0.03). B/Ca was determined using the Cameca 4f. Cultured coral analyses were normalized to multiple daily analyses of a Porites spp. coral standard (δ11B=24.8‰, B/Ca=0.364 mmol mol−1 (ref. 27)). The s.d. (1 σ) of bracketed standard analyses (n=13–19) each day was δ11B=1.7‰ and B/Ca=9%. Field coral analyses were normalized to the same Porites spp. standard, but a more homogenous Desmophyllum spp. cold-water coral chip was used to check for instrumental drift within and between days. The s.d. (1σ) of bracketed standard analyses (n=15–27) each day was δ11B=1.2‰ and B/Ca=2%. The precision (2σ) of the Porites spp. standard in each session was equivalent to ±0.02 pH units and ±3% B/Ca.
Estimation of partition coefficients
We estimated B(OH)4−/CO32−, B(OH)4−/HCO3− and B(OH)4−/(CO32−+HCO3−) aragonite partition coefficients from δ11B and B/Ca analyses of secondary aragonite cement in a Hawaiian fossil coral dated to 13.4 ky. We used δ11B to estimate coral pore fluid pH and assumed a porewater TA of 2,162±78 μmol kg−1 (n=4) based on repeat measurements of skeletal pore fluids in a modern coral18. Porewater [Ca] is similar to adjacent reefwaters (within 5%)18 and we assume that porewater [B] is the same as seawater (416 μmol kg−1) at the collection site of the fossil coral. We estimated B(OH)4−/CO32−, B(OH)4−/HCO3− and B(OH)4−/(CO32−+HCO3−) aragonite partition coefficients to be 0.283, 4.51 and 4.79, all × 10−3, respectively (Supplementary Table 1).
Calculation of ECF DIC parameters
The equilibrium constant, KB, and its pKB value were calculated28 from the known temperatures and salinity of the field sites and culture seawater. Mean annual salinity and temperature at the Jarvis and Hawaii reef sites are 35.5 and 27.4 °C (ref. 29) and 35.0 and 25.0 °C (ref. 17), respectively. Salinity and temperature in the culture system were 40.6 and 25.0 °C (ref. 22).
ECF pH was estimated from skeletal δ11B:

using the empirically determined αB (=1.0272)9 and assuming that the δ11BECF is the same as seawater (39.5‰).
We assumed that [B]ECF is the same as seawater, that is,

where S is the salinity28.
We used pHECF to estimate the [B(OH)4−]ECF:

B/Caaragonite equates to B/CO3 aragonite2− as Ca and C are equimolar in CaCO3. We used [B(OH)4−]ECF, B/Caaragonite and the relevant B/(co-precipitating DIC species) partition coefficients to estimate the concentration of the DIC species co-precipitating with B(OH)4− in the ECF. For example,


We used pHECF and the concentration of the DIC species co-precipitating with B (OH)4− in the ECF (that is, [CO32−]ECF in scenario 1, [HCO3−]ECF in scenario 2 and so on) to estimate all the other parameters in the ECF DIC system. DIC system parameters were calculated using CO2sys.xls30 using acidity constants K1 and K2 from Roy et al.31 and KHSO4 from Dickson32. ECF Ω was calculated using ECF [CO32−] and assuming that ECF [Ca2+] was similar to seawater19.
Additional information
How to cite this article: Allison, N. et al. Corals concentrate dissolved inorganic carbon to facilitate calcification. Nat. Commun. 5:5741 doi: 10.1038/ncomms6741 (2014).
References
Erez, J. Vital effect on the stable-isotope composition seen in foraminifera and coral skeletons. Nature 273, 199–202 (1978).
Schneider, K. & Erez, J. The effect of carbonate chemistry on calcification and photosynthesis in the hermatypic coral Acropora eurystoma. Limnol. Oceanogr. 51, 1284–1293 (2006).
Jury, C. P., Whitehead, R. F. & Szmant, A. M. Effects of variations in carbonate chemistry on the calcification rates of Madracis auretenra (= Madracis mirabilis sensu Wells, 1973): bicarbonate concentrations best predict calcification rates. Glob. Change Biol. 16, 1632–1644 (2010).
Furla, P., Galgani, I., Durand, I. & Allemand, D. Sources and mechanisms of inorganic carbon transport for coral calcification and photosynthesis. J. Exp. Biol. 203, 3445–3457 (2000).
Weber, J. N. & Woodhead, P. M. J. Carbon and oxygen isotope fractionation in the skeletal carbonate of reef-building corals. Chem. Geol. 6, 93–117 (1970).
Hemming, N. G. & Hanson, G. N. Boron isotopic composition and concentration in modern marine carbonates. Geochim. Cosmochim. Acta 56, 537–543 (1992).
Sen, S., Stebbins, J. F., Hemming, N. G. & Ghosh, B. Coordination environments of B-impurities in calcite and aragonite polymorphs - a B-11 mas NMR-study. Am. Mineral. 79, 819–825 (1994).
Tambutte, E. et al. Calcein labelling and electrophysiology: insights on coral tissue permeability and calcification. Proc. Biol. Sci. 279, 19–27 (2012).
Klochko, K., Kaufman, A. J., Yao, W. S., Bryne, R. H. & Tossell, J. A. Experimental measurement of boron isotope fractionation in seawater. Earth Planet. Sci. Lett. 248, 276–285 (2006).
Allen, K. A., Honisch, B., Eggins, S. M. & Rosenthal, Y. Environmental controls on B/Ca in calcite tests of the tropical planktic foraminifer species Globigerinoides ruber and Globigerinoides sacculifer. Earth Planet. Sci. Lett. 351-352, 270–280 (2012).
Dordas, C. & Brown, P. H. Permeability and mechanism of transport of boric acid across the plasma membrane of Xenopus laevis oocytes. Biol. Trace Elem. Res. 81, 127–139 (2001).
Venn, A. A. et al. Impact of seawater acidification on pH at the tissue-skeleton interface and calcification in reef corals. Proc. Natl Acad. Sci. USA 110, 1634–1639 (2012).
Krief, S. et al. Physiological and isotopic responses of scleractinian corals to ocean acidification. Geochim. Cosmochim. Acta 74, 4988–5001 (2010).
Gattuso, J. P., Allemand, D. & Frankignoulle, M. Photosynthesis and calcification at cellular, organismal and community levels in coral reefs: a review on interactions and control by carbonate chemistry. Am. Zool. 39, 160–183 (1999).
Gagnon, A. C. Coral calcification feels the acid. Proc. Natl Acad. Sci. USA 110, 1567–1568 (2013).
Venn, A. A., Tambutte, E., Holcomb, M., Allemand, D. & Tambutte, S. Live tissue imaging shows reef corals elevate pH under their calcifying tissue relative to seawater. PLoS ONE 6, e20013 (2011).
Allison, N. & Finch, A. A. EIMF. δ11B, Sr, Mg and B in a modern Porites coral: the relationship between calcification site pH and skeletal chemistry. Geochim. Cosmochim. Acta 74, 1790–1800 (2010).
Enmar, R. et al. Diagenesis in live corals from the Gulf of Aqaba. I. The effect on paleo-oceanography tracers. Geochim. Cosmochim. Acta 64, 3123–3132 (2000).
Al-Horani, F. A., Al-Moghrabi, S. M. & de Beer, D. The mechanism of calcification and its relation to photosynthesis and respiration in the scleractinian coral Galaxea fascicularis. Mar. Biol. 142, 419–426 (2003).
Adkins, J. F., Boyle, E. A., Curry, W. B. & Lutringer, A. Stable isotopes in deep-sea corals and a new mechanism for ‘vital effects’. Geochim. Cosmochim. Acta 67, 1129–1143 (2003).
McConnaughey, T. A. Sub-equilibrium oxygen-18 and carbon-13 levels in biological carbonates: carbonate and kinetic models. Coral Reefs 22, 316–327 (2003).
Allison, N., Cohen, I., Finch, A. A. & Erez, J. E.I.M.F. Controls on Sr/Ca and Mg/Ca in scleractinian corals: the effects of Ca-ATPase and transcellular Ca channels on skeletal chemistry. Geochim. Cosmochim. Acta 75, 6350–6360 (2011).
Burton, E. A. & Walter, L. M. Relative precipitation rates of aragonite and Mg calcite from seawater: temperature or carbonate ion control. Geology 15, 111–114 (1987).
Falini, G. et al. Control of aragonite deposition in colonial corals by intra-skeletal macromolecules. J. Struct. Biol. 183, 226–238 (2013).
Allison, N. & Finch, A. A. High resolution Sr/Ca records in modern Porites lobata corals: effects of skeletal extension rate and architecture. Geochem. Geophys. Geosyst. 5, Q05001 (2004).
Marriott, C. S., Henderson, G. M., Belshaw, N. S. & Tudhope, A. W. Temperature dependence of δ7Li, δ44Ca and Li/Ca during growth of calcium carbonate. Earth Planet. Sci. Lett. 222, 615–624 (2004).
Kasemann, S. A., Schmidt, D. N., Bijma, J. & Foster, G. L. In situ boron isotope analysis in marine carbonates and its application for foraminifera and palaeo-pH. Chem. Geol. 260, 138–147 (2009).
DOE. in Handbook of Methods for the Analysis of the Various Parameters of the Carbon Dioxide System in Seawater. Version 2. (eds Dickson, A. G. & Goyet, C.) (Department of Energy, ORNL/CDIAC-74, 1994).
Price, N. N., Martz, T. R., Brainard, R. E. & Smith, J. E. Diel variability in seawater pH relates to calcification and benthic community structure on coral reefs. PLoS ONE 7, e43843 (2012).
Pierrot, D. E. Lewis, D. & Wallace, W. R. MS Excel Program Developed for CO 2 System Calculations (Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, ORNL/CDIAC-105a, 2006).
Roy, R. N. et al. The dissociation constants of carbonic acid in seawater at salinities 5 to 45 and temperatures 0 to 45 °C. Mar. Chem. 44, 249–267 (1993).
Dickson, A. G. Standard potential of the reaction: AgCl2(s)+1/2H2(g)=Ag(s)+HCl(aq), and the standard acidity constant of the ion HSO4 in synthetic seawater from 273.15 to 318.15 K. J. Chem. Thermodyn. 22, 113–127 (1990).
Fagan, K. E. & Mackenzie, F. T. Air–sea CO2 exchange in a subtropical estuarine-coral reef system, Kaneohe Bay, Oahu, Hawaii. Mar. Chem. 106, 174–191 (2007).
Acknowledgements
This work was supported by the UK Natural Environment Research Council (awards NER/A/S/2003/00473 and NE/G015791/1 to N.A. and A.A.F.; NER/GR3/12021 to A.W.T.). Participation of J.E. and I.C. in this study was supported by DFG project Trion and the Israel Science Foundation (grants 870/05 and 551/10). Access to the ion probe was provided by NERC Scientific Services.
Author information
Authors and Affiliations
Contributions
N.A., J.E. and A.A.F. designed the study. Field samples were collected by N.A., A.A.F. and A.W.T. Coral culturing was performed by N.A., I.C. and J.E. SIMS was performed by N.A. and A.A.F. All authors contributed to the analysis of the results and to the writing of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Table 1 and Supplementary References (PDF 159 kb)
Rights and permissions
About this article
Cite this article
Allison, N., Cohen, I., Finch, A. et al. Corals concentrate dissolved inorganic carbon to facilitate calcification. Nat Commun 5, 5741 (2014). https://doi.org/10.1038/ncomms6741
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms6741
This article is cited by
-
Differences in carbonate chemistry up-regulation of long-lived reef-building corals
Scientific Reports (2023)
-
Coral calcification mechanisms in a warming ocean and the interactive effects of temperature and light
Communications Earth & Environment (2022)
-
Recent ocean acidification trends from boron isotope (δ11B) records of coral: Role of oceanographic processes and anthropogenic CO2 forcing
Journal of Earth System Science (2022)
-
Resolving the interactions of ocean acidification and temperature on coral calcification media pH
Coral Reefs (2021)
-
Inorganic carbon fluxes and perturbations by ocean acidification estimated using a data-constrained, process-based model of coral physiology
Marine Biology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.