Abstract
Animals can call on a multitude of sensory information to orient and navigate. One such cue is the pattern of polarized light in the sky, which for example can be used by birds as a geographical reference to calibrate other cues in the compass mechanism. Here we demonstrate that the female greater mouse-eared bat (Myotis myotis) uses polarization cues at sunset to calibrate a magnetic compass, which is subsequently used for orientation during a homing experiment. This renders bats the only mammal known so far to make use of the polarization pattern in the sky. Although there is currently no clear understanding of how this cue is perceived in this taxon, our observation has general implications for the sensory biology of mammalian vision.
Similar content being viewed by others
Introduction
When animals orient and navigate, they rely on a variety of sensory information. This can be the position of the sun or stars, strength and inclination of the Earth’s magnetic field, olfactory cues or the pattern of polarized light in the sky1. To achieve the best acuity of orientation and prevent mismatches, these different systems need to be calibrated against one another. Evidence from birds has shown that the pattern of polarized light in the sky is the primary geographical reference against which other cues are calibrated at certain life stages2,3,4,5. However, other results indicate that this mechanism might not be straightforward6,7,8,9. The use of polarization as an orientation cue is widespread in invertebrates. In vertebrates, birds, fish, reptiles and amphibians have been demonstrated to make use of polarized light, but not mammals10,11.
Bats use echolocation to orient and navigate. However, this sensory system is only sufficient for a short range of 5–50 m12,13. For longer range navigation bats must use different sensory input such as vision14,15. Furthermore, it has been demonstrated that bats can use the Earth’s magnetic field to orient and calibrate it against cues obtained at sunset16,17. These could be the position of the sun, or as used in birds, the pattern of polarized light. The polarization pattern is strongest at dusk and dawn. At this time a band of maximum polarization stretches 90° east from the position of the sun over the zenith to 90° west of the sun and gets weaker towards and away from the sun) (see natural polarization direction (PN) in Fig. 1a). On the horizon this band is aligned vertically, as is the e-vector of polarization2,10. To test the hypothesis that bats calibrate a magnetic compass with polarized light cues we conducted translocation experiments with 70 adult female greater mouse-eared bats (Myotis myotis). Prior to release, the bats could observe the sky at sunset in experimental boxes with different manipulations of the polarization pattern (natural (PN) versus 90° shifted polarization directions (PS) orientation of the band of maximum polarization).
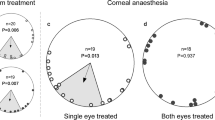
North (0°) is always at the top of the circles, except for a. The dashed circle depicts the Rayleigh significance threshold (P=0.05) and the arrows are the mean vector. The axial distribution of PS is shown by a double arrow. The triangle on the outside of the circles indicates the home direction. a is a 360° view of the sky with sunset and the experimental conditions. The dark bars represent the band of maximum polarization, mimicked by the filters. PN presents a natural polarization direction pattern, which is shifted 90° in PS. The bars on top of the experimental boxes symbolize the axis of vertical polarization in the windows. b are the control bats for RS1 (CC) that were untreated. c and d show the PN and PS data for release site 1 (RS1) and e and f for release site 2 (RS2).
We hypothesize that bats calibrate their magnetic compass at dusk using the direction of polarization. We predict that, when presented with a vertical polarization 90° left and right to the sunset (corresponding to the direction of the natural band of maximum polarization, Fig. 1a PN) the bats should depart in the home direction when tracked later at the release site. When the box is rotated 90° during sunset (Fig. 1a PS) the bats should also depart ~90° away from the home direction.
Results
For release site one (RS1) the results show that the group with PN had a mean angle significantly different from the home direction (home=224°, confidence interval test: P<0.01). However, it was not significantly different from the group of control bats that received no polarization treatment (control (CC), Mardia–Watson–Wheeler test, W=3.281, P=0.194), which also had a mean angle significantly different from the home direction (confidence interval test: P<0.01). Both were significantly oriented (Rayleigh’s test, CC: Z=8.376, P<0.0001; PN: Z=4.221, P=0.012, Fig. 1b,c). The group with the PS was significantly different from PN (Mardia–Watson–Wheeler test, W=6.936, P=0.031), albeit not significantly oriented (Rayleigh’s test, Z=1.637, P=0.192, Fig. 1d). For release site two (RS2) the mean angle of PN was not significantly different from the home direction (home=80°, confidence interval test: P>0.05) and also significantly oriented (Rayleigh’s test, Z=5.555, P=0.003). We again find that PS and PN are significantly different (Mardia–Watson–Wheeler test, W=6.58, P=0.037). In addition, the PS group at RS2 shows a significantly axially oriented behaviour (Rayleigh’s test, Z=3.335, P=0.033, Fig. 1e,f) and was significantly different from home (confidence interval test: P<0.01).
Discussion
These data are the first to support the hypothesis that bats use the polarization pattern in the sky for compass calibration. When the animals were presented with a polarization pattern that equals the natural direction (that is, vertical on the horizon in a North–South axis: PN), they were significantly oriented. At RS1 this is the same as the CC, albeit in both cases significantly different from the home direction, which is consistent with previous findings of homing at this site17. When treated with a 90° shifted polarization field, PS was significantly different from PN at both sites and at RS2 PS showed a significant axial shift of their vanishing bearing of about 90° in both directions to PN’s mean vector. This behaviour has been described before in experiments manipulating polarization cues and generally is linked to its non-directional nature, meaning that although the polarization pattern can be seen there is no polarity2,18. In our experiments, the sunset could have been used as a cue to incorporate this information, but apparently bats ignored this and weighed the polarization cue higher. This pattern provides a more reliable calibration point than the sun’s position. Even when the sun’s exact location is obscured by clouds, polarization can still give accurate information. In addition, this precise cue is visible long after the sun has set and is actually most prominent at dusk and dawn, and therefore perceivable when these bats emerge from their caves2. Bats are also fairly light sensitive, so a small amount of polarization might be sufficient for their calibration19. Recently, experiments have shown that dung beetles can even use the polarization pattern of the moon for orientation20,21.
Currently, it is unclear how bats could perceive the sky’s polarization pattern. In insects, polarization vision is coupled to clear morphological structures (dichroism) of the photoreceptors10,11. In vertebrates, oil droplets and double cones have been suggested as a mechanism for birds9,22. In fish there is also a hypothesized mechanism involving double cones23,24 and in anchovy the same morphological adaptation of the cone cell receptors is proposed to be involved in polarization vision25,26 as is in amphibians and reptiles27. The only other mammals known so far to be able to perceive polarization are humans. However, there is no known function and the mechanism is still unclear. The alignment of receptors in the macula has been proposed10, as has the role of the blue-cone distribution28. Little is known about the detailed retinal structure of greater mouse-eared bats, although they do not appear to possess a macula. Some bats however, seem to be sensitive in the ultraviolet range of light29,30, which also would be beneficial to perceive polarization31. Thus, both behavioural and physiological studies are required to elucidate how bats perceive the sky’s polarization pattern and exactly how they use it.
Methods
Animal models
Seventy non- or post-lactating adult female greater mouse-eared bats (M. myotis) were caught at their home roost (Orlova Chuka Cave, district Ruse) in Northeast Bulgaria, between 12 July and 7 August, 16–22 h before the experimental treatment and were kept at the nearby Siemers Bat Research Station, Tabachka. All experiments were carried out under the licence of the responsible Bulgarian authorities (the Bulgarian Ministry of Environment and Water, and Regional Inspectorate (RIOSV) Ruse, permit #465/29.06.2012).
Experimental treatment
For the experiments, bats were put in holding cages at a treatment site 1.3 km away from their cave and offered a clear view of the horizon in all directions from 22 min before until 75 min after sunset (when the last visible post-sunset glow had disappeared). All experimental evenings generally had clear sky and always had a visible sunset with only the exceptional cloud coming up. Bats were placed in holding cages which consisted of an inner (120 × 120 × 63 mm) and outer (148 × 148 × 72 mm) cardboard box. The inner holding box had windows (98 × 47 mm) covered with plastic mesh (4 mm square). The outer experimental box also had windows (108 × 52 mm), covered with an outer layer of a pseudo-depolarizing filter (90% depolarization with a 10–15% reduction of light intensity for a range of 400–800 nm)6, effectively eliminating natural polarization. The windows further had an inner layer which was a polarizing filter (linear polarizer P500, 3Dlens Corporation Taiwan, ultraviolet block, transmittance: 43%, polarizing: efficiency 99.9% at 380–700 nm)2. The polarization direction of the inner filter was either vertically or horizontally oriented, with opposite sides having the same direction (Fig. 2). The boxes were oriented either with the vertically polarized windows 90° away from the sun (in a North–South axis), corresponding to the natural situation (PN), or they were shifted 90° so that now the horizontally polarized windows were oriented North–South (PS) (Fig. 1a). Bats were also kept in a double-wrapped magnetic Helmholtz coil with current antiparallel (resulting in no change of the natural magnetic field), as they served as a control group for another experiment (see Supplementary Data 1 for details of release nights).
Testing
After the treatment, the bats were translocated to either of two release sites, with RS1 23.6 km north–north–east and RS2 20.4 km south–west–west away from the treatment site. Both sites were flat fields, offering a clear line of sight in all directions. Bats were then fed 20–40 mealworm larvae (Tenebrio molitor) and watered ad libitum. They were equipped with radio transmitters (BioTrack PicoPip AG379, 0.44 g) and released singly starting at 0100 hours. The bats were tracked from the roof of a car using a radio receiver (AR8200 III, AOR) connected to a five-element Yagi antenna which was mounted on a 4 m pole, thus reaching ~6 m in height. The release direction of the bats was chosen randomly and the only person tracking them was blind to the treatment. Each bat was tracked until the radio signal could no longer be heard and after at least 1 min of silence the direction was noted as the vanishing bearing. Each evening the same number of bats for PN and PS were released with 15 each for RS1 and 16 PN and 17 PS for RS2. For RS1 we added another control group which received no polarization treatment (CC, n=14).
Statistical analysis
Mean bearings and vector lengths of each group were calculated using the Oriana 4.0 circular statistics software package. All groups were tested for significant orientation using the Rayleigh’s test. Tests for significant differences between groups were performed using the Mardia–Watson–Wheeler test. Previous studies have found that in polarization experiments, due to the fact that the cue is axial the treatment group often reacts bimodally2,18. Thus, we applied a decision rule whereby for each group, if the vector length, raxial>runimodal, data would be analyzed as axial32.
Additional information
How to cite this article: Greif, S. et al. A functional role of the sky’s polarization pattern for orientation in the greater mouse-eared bat. Nat. Commun. 5:4488 doi: 10.1038/ncomms5488 (2014).
References
Papi, F. Animal Homing Chapman & Hall (1992).
Muheim, R., Phillips, J. B. & Akesson, S. Polarized light cues underlie compass calibration in migratory songbirds. Science 313, 837–839 (2006).
Muheim, R., Phillips, J. B. & Deutschlander, M. E. White-throated sparrows calibrate their magnetic compass by polarized light cues during both autumn and spring migration. J. Exp. Biol. 212, 3466–3472 (2009).
Muheim, R., Moore, F. R. & Phillips, J. B. Calibration of magnetic and celestial compass cues in migratory birds--a review of cue-conflict experiments. J. Exp. Biol. 209, 2–17 (2006).
Phillips, J. & Moore, F. Calibration of the sun compass by sunset polarized light patterns in a migratory bird. Behav. Ecol. Sociobiol. 31, 189–193 (1992).
Åkesson, S. & Bäckman, J. Orientation in pied flycatchers: the relative importance of magnetic and visual information at dusk. Anim. Behav. 57, 819–828 (1999).
Schmaljohann, H., Rautenberg, T., Muheim, R., Naef-Daenzer, B. & Bairlein, F. Response of a free-flying songbird to an experimental shift of the light polarization pattern around sunset. J. Exp. Biol. 216, 1381–1387 (2013).
Wiltschko, R., Munro, U., Ford, H. & Wiltschko, W. Contradictory results on the role of polarized light in compass calibration in migratory songbirds. J. Ornithol. 149, 607–614 (2008).
Muheim, R. Behavioural and physiological mechanisms of polarized light sensitivity in birds. Philos. Trans. R Soc. Lond. B Biol. Sci. 366, 763–771 (2011).
Horváth, G. & Varjú, D. Polarized Light in Animal Vision Springer (2004).
Marshall, J. & Cronin, T. W. Polarisation vision. Curr. Biol. 21, R101–R105 (2011).
Stilz, W.-P. & Schnitzler, H.-U. Estimation of the acoustic range of bat echolocation for extended targets. J. Acoust. Soc. Am. 132, 1765–1775 (2012).
Boonman, A., Bar-On, Y., Yovel, Y. & Cvikel, N. It’s not black or white-on the range of vision and echolocation in echolocating bats. Front. Physiol. 4, 248 (2013).
Williams, T., Williams, J. & Griffin, D. The homing ability of the neotropical bat Phyllostomus hastatus, with evidence for visual orientation. Anim. Behav. 14, 468–473 (1966).
Tsoar, A. et al. Large-scale navigational map in a mammal. Proc. Natl Acad. Sci. USA 108, E718–E724 (2011).
Holland, R. A., Thorup, K., Vonhof, M. J., Cochran, W. W. & Wikelski, M. Bat orientation using Earth’s magnetic field. Nature 444, 702 (2006).
Holland, R. A., Borissov, I. & Siemers, B. M. A nocturnal mammal, the greater mouse-eared bat, calibrates a magnetic compass by the sun. Proc. Natl Acad. Sci. USA 107, 6941–6945 (2010).
Beltrami, G., Bertolucci, C., Parretta, A., Petrucci, F. & Foà, A. A sky polarization compass in lizards: the central role of the parietal eye. J. Exp. Biol. 213, 2048–2054 (2010).
Joshi, D. & Chandrashekaran, M. K. Daylight dimmer than starlight entrains the circadian rhythm of a bat. Naturwissenschaften 69, 192–193 (1982).
Dacke, M., Nilsson, D.-E., Scholtz, C. H., Byrne, M. & Warrant, E. J. Insect orientation to polarized moonlight. Nature 424, 33–34 (2003).
Dacke, M., Byrne, M. J., Baird, E., Scholtz, C. H. & Warrant, E. J. How dim is dim? Precision of the celestial compass in moonlight and sunlight. Philos. Trans. R Soc. Lond. B Biol. Sci. 366, 697–702 (2011).
Young, S. R. & Martin, G. R. Optics of retinal oil droplets: a model of light collection and polarization detection in the avian retina. Vision Res. 24, 129–137 (1984).
Cameron, D. A. & Pugh, E. N. Double cones as a basis for a new type of polarization vision in vertebrates. Nature 353, 161–164 (1991).
Roberts, N. W. & Needham, M. G. A mechanism of polarized light sensitivity in cone photoreceptors of the goldfish Carassius auratus. Biophys. J. 93, 3241–3248 (2007).
Flamarique, I. N. & Hárosi, F. I. Visual pigments and dichroism of anchovy cones: a model system for polarization detection. Vis. Neurosci. 19, 467–473 (2002).
Novales Flamarique, I. Unique photoreceptor arrangements in a fish with polarized light discrimination. J. Comp. Neurol. 519, 714–737 (2011).
Hamasaki, D. & Eder, D. inHandbook of Sensory Physiology ed. Crescitelli F. 497–548Springer (1977).
Le Floch, A., Ropars, G., Enoch, J. & Lakshminarayanan, V. The polarization sense in human vision. Vision Res. 50, 2048–2054 (2010).
Müller, B. et al. Bat eyes have ultraviolet-sensitive cone photoreceptors. PLoS ONE 4, e6390 (2009).
Zhao, H. et al. The evolution of color vision in nocturnal mammals. Proc. Natl Acad. Sci. USA 106, 8980–8985 (2009).
Barta, A. & Horváth, G. Why is it advantageous for animals to detect celestial polarization in the ultraviolet? Skylight polarization under clouds and canopies is strongest in the UV. J. Theor. Biol. 226, 429–437 (2004).
Batschelet, E. Circular Statistics in Biology Academic Press (1981).
Acknowledgements
We thank Holger Goerlitz for discussion and company in the field, as well as the rest of the 2013 field crew. We also thank Rachel Muheim for valuable comments at the planning stage. We are grateful to the Directorate of the Rusenski Lom Nature Park (director Milko Belberov) and the Bulgarian Bat Research and Conservation Group for cooperation and support. The study was funded by a Natural Environment Research Council grant (NE/K000381/1) to R.A.H. and by the Max Planck Society.
Author information
Authors and Affiliations
Contributions
S.G. and R.A.H. designed the study. All authors performed the experiments. S.G. and R.A.H. analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Data 1
Raw data of bat releases (XLS 47 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Greif, S., Borissov, I., Yovel, Y. et al. A functional role of the sky’s polarization pattern for orientation in the greater mouse-eared bat. Nat Commun 5, 4488 (2014). https://doi.org/10.1038/ncomms5488
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms5488
This article is cited by
-
Editors’ Choice 2023
Communications Engineering (2023)
-
An artificial neural network explains how bats might use vision for navigation
Communications Biology (2022)
-
Artificial Lighting at Night in Estuaries—Implications from Individuals to Ecosystems
Estuaries and Coasts (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.