Abstract
Genetically engineered mouse models that employ site-specific recombinase technology are important tools for cancer research but can be costly and time-consuming. The CRISPR-Cas9 system has been adapted to generate autochthonous tumours in mice, but how these tumours compare to tumours generated by conventional recombinase technology remains to be fully explored. Here we use CRISPR-Cas9 to generate multiple subtypes of primary sarcomas efficiently in wild type and genetically engineered mice. These data demonstrate that CRISPR-Cas9 can be used to generate multiple subtypes of soft tissue sarcomas in mice. Primary sarcomas generated with CRISPR-Cas9 and Cre recombinase technology had similar histology, growth kinetics, copy number variation and mutational load as assessed by whole exome sequencing. These results show that sarcomas generated with CRISPR-Cas9 technology are similar to sarcomas generated with conventional modelling techniques and suggest that CRISPR-Cas9 can be used to more rapidly generate genotypically and phenotypically similar cancers.
Similar content being viewed by others
Introduction
Genetically engineered mouse models (GEMMs) are important tools for studying cancer in vivo1,2. First-generation GEMMs relied on transgenic germline manipulation to express oncogenes. Subsequent GEMMs applied homologous recombination by targeting tumour suppressor genes in embryonic stem (ES) cells by introducing specific mutations into a defined genetic locus. When this approach was modified to permit regulation by site-specific recombinases (SSR), such as Cre recombinase, investigators gained spatial and temporal control over the development of cancer in mice3. We and many other laboratories have successfully used this approach to gain important insights into cancer and to study the response of primary tumours to cancer therapy4,5. However, the process of generating GEMMs is costly and time consuming, as it includes targeting ES cells, breeding mice, and genotyping the progeny. These drawbacks are compounded when more complex models are desired that involve conditional, inducible, or multiple alleles.
The discovery and adaptation of the prokaryotic clustered regularly interspaced short palindromic repeats (CRISPR) system for genome editing in eukaryotic cells and organisms, including mice, has expanded the in vivo disease modelling armamentarium6,7,8,9,10,11,12. In particular, CRISPR-Cas9 genome engineering has been utilized to create mouse models of cancers of the pancreas, liver and lung, among other organs10,13,14,15,16,17,18,19. Developing mouse models of soft tissue sarcoma, however, has remained challenging. First, soft tissue sarcomas arise from connective tissue and encompass a broad range of subtypes. For many sarcoma subtypes, the progenitor cells remain unknown and targeting the correct progenitor has been a barrier to modelling distinct sarcoma histologies in mice20,21,22. Second, soft tissue sarcomas are genetically diverse. Some sarcomas harbour simple translocations such as the t(11;22)(q24;q12) translocation commonly found in Ewing sarcoma, whereas others have complex genetics such as in malignant peripheral nerve sheath tumour (MPNST) and undifferentiated pleomorphic sarcoma (UPS)23,24. Although spatially and temporally restricted mouse models of UPS and MPNST have been generated using Cre-loxP technology3,25, it has been challenging to generate robust preclinical models for some sarcomas such as Ewing sarcoma23. As sarcomas are a rare and under-studied group of tumours, there is a need for in vivo models that accurately recapitulate this spectrum of cancers. Here we apply the CRISPR-Cas9 system to generate autochthonous UPS in a GEMM and MPNST in wild-type 129/SvJ mice.
The use of CRISPR-Cas9 to generate models of cancer has increased due to its utility in rapidly editing somatic cells. As CRISPR-Cas9 mouse models become more prevalent in preclinical research, it is important to carefully evaluate how these models compare with conventional models such as those generated using SSR technology. For example, traditional cancer models generated using Cre recombinase can provide temporal and spatial control for tumour development, but require a relatively long and costly process to genetically engineer the germline DNA. With CRISPR-Cas9 technology, somatic mutations can be quickly generated to initiate cancers with spatial and temporal control. Yet, as Cas9 is an efficient endonuclease, unintended off-target genomic changes may have an impact on in vivo phenotypes. To our knowledge, cancer mouse models generated by CRISPR-Cas9 and SSRs have not been systematically compared. Here we compare two mouse models of UPS generated with either Cre-loxP or CRISPR-Cas9 technology and find similar histology, growth kinetics and mutational profiles between these models.
Results
A combined Cre-loxP-CRISPR-Cas9 system for genome editing
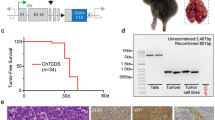
We have previously shown that intramuscular delivery of an adenovirus expressing Cre recombinase into mice with conditional mutations in Kras and Trp53 results in the formation of soft tissue sarcomas at the site of injection3. To recapitulate this model using a combined Cre-loxP and CRISPR-Cas9 system, we crossed KrasLSL-G12D/+ (K) mice with Rosa26LSL-Cas9-EGFP/+ (C) mice to generate KrasLSL-G12D/+; Rosa26LSL-Cas9-EGFP/+ (KC) mice. KC mice conditionally express oncogenic Kras and Cas9 in the presence of Cre recombinase through removal of upstream floxed STOP cassettes (LSL)10,26. We constructed the pX333-sgTrp53-Cre (pX333-P-Cre) plasmid to deliver both Cre recombinase and a single-guide RNA (sgRNA) targeting the mouse Trp53 exon 7 (Fig. 1a and Supplementary Table 1). To assess the applicability of a combined Cre-loxP and CRISPR-Cas9 system for oncogenic transformation in vitro, mouse embryonic fibroblasts (MEFs) from K mice, C mice and KC mice were transiently transfected with the pX333-P-Cre plasmid and subjected to DNA analysis, soft agar assay and an in vivo allograft experiment (Fig. 1b). PCR analysis of genomic DNA from transfected K MEFs revealed activation of KrasG12D alone, from transfected C MEFs revealed activation of Cas9 alone and from transfected KC MEFs revealed activation of both KrasG12D and Cas9 (Fig. 1c). In addition, insertions or deletions (indels) in Trp53 were detected in transfected C MEFs and KC MEFs by Surveyor nuclease assay, but not in K MEFs as expected (Fig. 1c). Activation of oncogenic Kras and deletion of Trp53 were both required for anchorage-independent growth (Fig. 1d) and to form tumours resembling sarcoma following intramuscular injection into nude mice (Fig. 1e). Thus, these results show that a combined Cre-loxP and CRISPR-Cas9 system, which activates KrasG12D and mutates Trp53 in vitro, is sufficient for transformation.
(a) The pX333 plasmid was modified to express Cre. A sgRNA to target Trp53 was cloned into the plasmid following a U6 promoter. (b) MEFs from KrasLSL-G12D/+ (K), Rosa26LSL-Cas9-EGFP/+ (C) or KrasLSL-G12D/+; Rosa26LSL-Cas9-EGFP/+ (KC) mice were harvested and cultured. MEFs were transiently transfected with pX333-sgTrp53-Cre and subsequently DNA analysis, soft agar studies and an allograft study were performed. (c) PCR shows that transient transfection of pX333-sgTrp53-Cre in MEFs resulted in recombination of KrasLSL-G12D/+ and Rosa26LSL-Cas9-EGFP/+ (top two panels). Surveyor assay (bottom panel) shows that pX333-sgTrp53-Cre efficiently generates indels in the Trp53 gene. (d) MEFs transfected with pX333-sgTrp53-Cre were subjected to a soft agar assay. When KC MEFs were transfected with the plasmid, deletion of Trp53 and activation of the conditional Kras mutation resulted in anchorage-independent growth. The result is representative of at least two different experiments. Furthermore, when these transformed cells were injected into nude mice (n=4) (e), they all formed tumours that showed a spindle cell morphology. Scale bars, 100 μm.
Generation of primary soft tissue sarcomas using CRISPR-Cas9
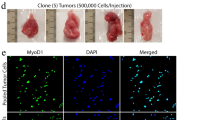
Having achieved transformation by transient transfection of the pX333-P-Cre plasmid in KC MEFs in vitro, we next explored the possibility of generating primary sarcomas in KC mice by intramuscular injection of Adeno-sgTrp53-Cre (Ad-P-Cre), an adenovirus expressing sgTrp53 and Cre recombinase (Fig. 2a). After transduction of Ad-P-Cre adenovirus into KC MEFs, KrasG12D and Cas9 were activated and Trp53 indels were detected by Surveyor nuclease assay (Supplementary Fig. 1A). We then injected K mice (n=10), C mice (n=10), and KC mice (n=20) intramuscularly with Ad-P-Cre adenovirus. Following Ad-P-Cre injection, we observed tumours in 100% of KC mice with tumour onset as early as 7.3 weeks (Fig. 2b). Of note, the time frame of Ad-P-Cre adenovirus-initiated tumour development (median 9.6 weeks) was similar to that of tumours generated in KrasLSL-G12D/+; Trp53Flox/Flox (KP) mice by intramuscular injection of Ad-Cre (median 11.3 weeks)3. The virus did not generate sarcomas in mice harbouring only KrasLSL-G12D/+ or only the Rosa26LSL-Cas9-EGFP/+ allele (Fig. 2b). PCR confirmed KrasG12D activation and Surveyor assay showed evidence of Trp53 indels in three cell lines derived from Ad-P-Cre-initiated tumours (Fig. 2c). Trp53 complete knockout was also confirmed by western blotting, which showed no Trp53 expression induced by doxorubicin (Supplementary Fig. 1B)27. Interestingly, wild-type Kras, as well as activated Cas9, were undetectable in these tumour cells (Fig. 2c, Supplementary Fig. 1C). Loss of both the wild-type Kras allele and the adjacent Cas9 allele may be selected for during sarcomagenesis because the wild-type Kras allele can act as a tumour suppressor in the presence of an oncogenic Kras that drives transformation and tumorigenesis28,29,30. Similar to sarcomas generated in Ad-Cre injected KP mice31, immunohistochemical analysis of 12 Ad-P-Cre-induced tumours demonstrated that the majority of tumours (n=10) are UPS without myogenic staining, whereas a minority (n=2) were myogenic UPS (Fig. 2d,e).
(a) KrasLSL-G12D/+ (K), Rosa26LSL-Cas9-EGFP/+ (C), or KrasLSL-G12D/+; Rosa26LSL-Cas9-EGFP/+ (KC) mice received intramuscular injections of an adenovirus carrying the plasmid. (b) Adenoviral delivery of sgTrp3-Cre resulted in efficient primary sarcoma formation by activating conditional expression of the mutant Kras and Cas9 alleles and delivering a sgRNA to target Trp53. (c) PCR analysis and Surveyor assay showing recombination of KrasLSL-G12D and Rosa26LSL-Cas9-EGFP, as well as indels within the Trp53 gene, respectively. (d) Twelve of the primary tumours generated via adenoviral plasmid delivery were subject to histopathological analysis with staining for myogenic markers MyoD and myogenin. (e) The majority of tumours resembled UPS, whereas a minority were consistent with a myogenic UPS phenotype. Scale bars, 500 μm.
Assessment of clonality and mutations
Non-homologous end joining repair following Cas9-induced double-strand breaks results in multiple indels in the targeted Trp53 exon 7 (ref. 8). We assessed the clonality of sarcomas generated by Ad-P-Cre injection into KC mice by quantifying the number of unique mutations in exon 7 of Trp53 in each tumour. To define the indels in Trp53 in primary sarcomas, we performed Sanger sequencing of cell lines derived from each independent primary sarcoma (n=4). Sanger sequencing demonstrated that no wild-type Trp53 alleles were retained and indicated that three cell lines had at most two unique alterations for the two Trp53 alleles (Fig. 3a), suggesting that these sarcoma cell lines arose from a single clone that underwent genome editing at both Trp53 alleles. Interestingly, four distinct indels in the Trp53 site were detected in one cell line (2995), suggesting that this sarcoma cell line either contained at least two clones or arose because of tetraploidization before mutagenesis, which is a frequent event in tumorigenesis32,33,34. This high frequency of biallelic modification could reflect high efficiency of on-target CRISPR-Cas9 gene modification, such that if one allele is modified it is highly likely for the other allele to be modified as well. Alternatively, cells with both Trp53 alleles altered may be more likely or faster to contribute to sarcomagenesis. We investigated the first possibility by assessing the efficiency of CRISPR-Cas9 editing of a site on chromosome 17 that is a perfect match for the sgRNA that we used to target Trp53 and that should not contribute to tumorigenesis. This site on chromosome 17 is an intergenic region located more than 125 kb away from the nearest protein coding gene (Supplementary Fig. 2A). Surveyor assay of the on-target chromosome 17 site showed evidence of indels in the Cas9 recognition site (Supplementary Fig. 2B). To define the indels at this site in primary sarcomas, we performed Sanger sequencing of the same four independent sarcoma cell lines (n=4) from which we analysed Trp53 modification. Sanger sequencing demonstrated that no wild-type sequence at the on-target chromosome 17 site was retained and indicated that each cell line had at most two unique alterations for this on-target chromosome 17 site (Fig. 3c). These data demonstrate that the high efficiency of genome editing by sgTrp53 is not restricted to genes required for transformation, suggesting that the CRISPR-Cas9 system could be utilized to target other genes to test their ability to modify sarcoma development and other phenotypes. As clonal analysis of derivative cell lines may introduce an additional level of ex vivo clonal selection, we also performed deep sequencing of Trp53 and the on-target chromosome 17 sites in primary sarcomas (n=5). Several deletions in the Trp53 and on-target chromosome 17 sites identified by Sanger sequencing of the cell lines were too large to be detected by deep sequencing. This limitation of our deep sequencing was most likely to be due to primer design, which failed to amplify the targeted loci in the presence of a large indel that may have eliminated a complementary primer sequence required for PCR amplification. We still only identified a maximum of two distinct indels, defined as >1% of total reads by deep sequencing, in Trp53 (Fig. 3b and Supplementary Table 2). Deep sequencing of the on-target chromosome 17 site also showed a maximum of two distinct indels in four out of five primary sarcomas (Fig. 3d and Supplementary Table 2). However, three distinct indels in the on-target chromosome 17 site were detected in one tumour (2995). This finding further indicated that this sarcoma contained either multiple clones or underwent tetraploidization before mutagenesis.
(a) Sanger sequencing of the Trp53 gene in tumours generated in KC mice after adenoviral delivery of pX333-sgTrp53-Cre. Most of the sequenced alleles demonstrated deletions but there was a single nucleotide insertion observed in mouse 2981- allele 1. (b) Targeted deep sequencing of the Trp53 gene in tumours generated in KC mice. (Blue=sgTrp53; green=PAM; red=insertion/deletion). (c) Sanger sequencing of the on-target chromosome 17 site in tumours generated in KC mice after adenoviral delivery of pX333-sgTrp53-Cre. Most of the sequenced alleles demonstrated deletions but there was a single nucleotide insertion observed in mouse 2981- allele 1 and two nucleotides insertion observed in mouse 2995 allele 2. (d) Targeted deep sequencing of the on-target chromosome 17 site in tumours generated in KC mice. (Blue=sgTrp53; green=PAM; red=insertion/deletion).
Although Cas9 precisely cleaved the targeted Trp53 region and on-target chromosome 17 site with perfect complementarity to the sgRNA to produce site-specific indels in each tumour, several studies have shown that Cas9 activity may also result in off-target mutations in other regions of the genome35,36. We performed targeted deep sequencing to profile the top ten predicted off-target sites of the Trp53 sgRNA37. No significant off-target indels were observed in five Ad-P-Cre-induced sarcomas when compared to normal muscle from the same mouse as a control (Supplementary Table 3). Only a single tumour contained an edited exonic off-target site (Celsr1). In addition, the Surveyor assay identified DNA mismatches in the tumour DNA that further Sanger sequencing determined to be SNPs rather than mutations (Supplementary Fig. 3). We analysed the remaining nine non-exonic off-target sites by Surveyor assay and Sanger sequencing (Supplementary Fig. 4). DNA mismatches were only detected in one off-target site by Surveyor assay, which were also confirmed as SNPs in both the tumour and normal DNA by Sanger sequencing. Taken together, these results indicate that Cas9 specifically cleaves the targeted region to produce site-specific indels in each tumour without causing detectable off-target mutations in other regions of the genome.
Primary sarcoma generation via in vivo electroporation
Virus-mediated delivery was very efficient for tumour generation and matched the kinetics of KP tumours generated using Ad-Cre only (Fig. 2b). To further simplify this approach, we injected the pX333-P-Cre plasmid directly into muscle and observed tumours in 4 out of 15 KC mice with tumours forming as early as 13.1 weeks (Fig. 4a). The time frame for tumour development via naked plasmid injection was both delayed and less efficient compared to Ad-P-Cre virus delivery. However, electroporation of plasmid efficiently generated tumours (80% penetrance after median 10.8 weeks, Fig. 4) with similar kinetics and histology as delivery of Ad-P-Cre (median 9.6 weeks, Fig. 2b,d). In vivo electroporation (IVE) of naked plasmid pcDNA3-EGFP was ∼38 times more efficient for enhanced green fluorescent protein (EGFP) delivery to muscle fibres compared with adenovirus delivery of EGFP, but this increased efficiency did not affect the penetrance or the kinetics of sarcoma formation (Supplementary Fig. 5). To further optimize this system, we generated KrasLSL-G12D/+; Rosa26loxP-Cas9-EGFP/+ mice (K-loxP-C) that express Cas9 endogenously. After electroporation with pX333-P-Cre plasmid, the K-loxP-C mice developed sarcomas with 100% penetrance (Fig. 4). The time to tumour onset was similar to Ad-P-Cre tumours in KC mice, but occurred within a narrower window of time ranging from 7.0 to 10.7 weeks (median 7.4 weeks, Fig. 4).
(a) K, C and KC mice received intramuscular injections of the naked pX333-sgTrp53-Cre and K, C, KC and K-loxP-C mice received intramuscular injections of the naked pX333-sgTrp53-Cre with IVE. (b) Ten of the primary tumours generated via IVE were subject to histopathological analysis with haematoxylin and eosin staining. Scale bars, 200 μm.
Primary MPNST generation in wild-type mice with CRISPR-Cas9
We next investigated whether other mouse models of sarcoma could be generated using CRISPR-Cas9 technology. MPNSTs are aggressive sarcomas that arise from cells of the peripheral nerve sheath. Loss-of-function mutations in the Ras regulatory gene neurofibromin 1 (Nf1) and the tumour suppressor Cdkn2a (also known as p16/p19 or Ink4a/Arf) are commonly found in MPNSTs38. We have previously described a Cre-loxP-driven model of MPNST generated in Nf1Flox/Flox; Ink4a/ArfFlox/Flox mice through Ad-Cre injection into the sciatic nerve25. In this model, the median time to tumour onset was 17.6 weeks (ranging from 8.9 weeks to 38.9 weeks). Mice with mutations in Nf1 and Trp53 in cis on chromosome 11 (Nf1+/−; p53+/−) also develop MPNSTs through spontaneous loss of heterozygosity of the wild-type Nf1 and Trp53 alleles39. Therefore, we used sgTrp53 in combination with a sgRNA targeting exon 7 of Nf1 (sgNf1) (Supplementary Table 1), to determine whether the CRISPR-Cas9 system could generate MPNSTs in wild-type 129/SvJ mice. We constructed an adenoviral vector expressing sgNf1, sgTrp53 and Cas9 (Ad-NP-Cas9), and packaged the construct into adenoviral particles (Fig. 5a). Nf1 and Trp53 gene editing was achieved rapidly and efficiently upon transduction of Ad-NP-Cas9 adenovirus into NIH-3T3 mouse fibroblasts (Supplementary Fig. 6A). Infection of wild-type MEFs with Ad-NP-Cas9 adenovirus was sufficient for transformation, as determined by colony formation in soft agar (Supplementary Fig. 6B) and tumour formation in nude mice allografts (Supplementary Fig. 6C). To determine whether MPNSTs could be generated in wild-type mice using CRISPR-Cas9, Ad-NP-Cas9 adenovirus was injected into the sciatic nerves of wild-type 129/SvJ mice (n=12). Mice were followed for 150 days after injection and we detected tumours in 10 of 12 mice (median time to tumour 14.4 weeks, range 10.3–36.6 weeks) (Fig. 5b). At 23 and 32.1 weeks, the other 2 mice were found dead with no tumours detected. Nf1 and Trp53 indels were detected by Surveyor assay in three of three cell lines derived from these tumours (Fig. 5c), and Nf1 and Trp53 knockout was confirmed by western blotting (Supplementary Fig. 6D). Furthermore, we performed histological analysis on five tumours revealing that each was an MPNST, with one tumour also containing myogenic features (Fig. 5d,e). Taken together, these data show that MPNSTs can be generated in wild-type mice by applying CRISPR-Cas9 technology to target both Nf1 and Trp53 alleles.
(a) The px333 plasmid was modified by cloning a sgRNA targeting Nf1 into the plasmid following the first U6 promoter and a sgRNA targeting Trp53 following the second U6 promoter. (b) Wild-type 129/SvJ mice received sciatic nerve injections of an adenovirus carrying CRISPR components, and the mice were monitored for tumour formation. The formation of tumours was observed at the site of injection in these wild-type mice and (c) Surveyor analysis revealed indels in Nf1 and Trp53. (d) Haematoxylin and eosin histology and (e) positive S100 staining were consistent with MPNST. Scale bars, 100 μm.
Comparison of CRISPR-Cas9 and Cre-mediated primary sarcomas
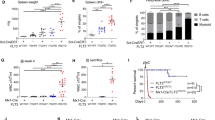
The primary sarcomas initiated by CRISPR-Cas9-Cre-loxP and Cre-loxP alone resembled each other on haematoxylin and eosin-stained sections (Fig. 6a) and had similar growth kinetics as measured by time to tumour tripling (median 8 days, Fig. 6b). To profile the mutational load and copy number variation (CNV) using an unbiased approach, we performed whole exome sequencing of primary mouse sarcomas (Fig. 6c,d). We compared the mutational load, which we defined as the number of non-synonymous somatic mutations per megabase (MB) of DNA, from tumours generated in KC mice following Ad-P-Cre injection (n=5) and tumours generated in KP mice following Ad-Cre injection (n=7). We also compared the amplifications and deletions to assess global CNV in these tumours. Overall, there were no significant differences in mutational load and CNV between sarcomas generated with or without CRISPR-Cas9 technology (Fig. 6c,d). Therefore, these results demonstrate that a combined Cre-loxP and CRISPR-Cas9 system that activates KrasG12D and deletes Trp53 in KC mice is sufficient to result in primary soft tissue sarcomas with mutational load and CNV similar to an established Cre-loxP tumour model.
(a) Ten of the primary tumours generated via Cre-loxP or a combined CRISPR-Cas9-Cre-loxP system were subject to histopathological analysis with haematoxylin and eosin staining. (b) Comparison of the days to tripling of primary sarcomas generated with Cre-loxP (n=15) and the combined CRISPR-Cas9-Cre-loxP (n=20) system. Scale bars, 200 μm. The non-synonymous mutational load (c) and CNVs (d) of primary sarcomas generated with Cre-loxP (n=7) and the combined CRISPR-Cas9-Cre-loxP (n=5) system was determined using whole exome sequencing of tumour DNA (Error bars=s.e.m.).
Discussion
Here we report, to the best of our knowledge, the first application of CRISPR-Cas9-mediated mutagenesis to generate autochthonous mouse models of sarcoma in GEMMs and wild-type mice. We generated primary UPS tumours in vivo using either Cre-loxP only or a combined Cre-loxP and CRISPR-Cas9 system. This hybrid system can be applied to existing mouse models of sarcoma for functional studies of genes mutated in human soft tissue sarcomas. Importantly, we compared the histopathology, tumour growth kinetics and mutational profiles of the tumours generated using these two systems, to determine that CRISPR-Cas9 generates similar mouse models of sarcoma compared to traditional SSR technology. In addition, we generated MPNSTs in wild-type mice with Cas9 and sgRNAs targeting Nf1 and Trp53. These models decrease the expense and time to generate primary sarcomas, which should expand the opportunity to employ GEMMs by more investigators in cancer research.
Primary soft tissue sarcomas generated in KC mice using the combined Cre-loxP and CRISPR-Cas9 technology recapitulate many features of the mouse UPS model generated in KP mice by Cre-loxP alone. Therefore, similar to the KP sarcoma model, we anticipate that sarcomas generated by CRISPR-Cas9 could also be used as a platform to study lung metastasis, to investigate mechanisms of tumour response to cancer therapy and to test new imaging agents3,40,41,42,43. We showed that one sgRNA efficiently targeted Cas9 to both Trp53 alleles and both on-target chromosome 17 alleles, which suggests that this system will also be useful for editing potential tumour modifying genes identified in human sarcomas to test their role during sarcomagenesis. This system can also be used to study tumour clonality, as unique indels are generated by non-homologous end joining after gene editing by Cas9. It is important to note that variation between two indels may be unique in some cases, but are certainly non-random44. Consequently, targeting only one locus makes it impossible to assess clonality with any degree of certainty due to the probability of generating identical sets of indels in two or more independent clones due to indel bias. By targeting several loci and generating indels across multiple loci, a unique indel barcode can be generated endogenously. To demonstrate the feasibility of this application, we employed an sgRNA with two different perfect on-target sites, one in the Trp53 locus and another locus on chromosome 17, as a method of multiplexed generation of four unique indels per cell (one indel per targeted allele) that represents a specific and endogenous genetic barcode to follow clonality during soft tissue sarcoma development. Interestingly, the Sanger sequencing analysis of cell lines from three independent sarcomas suggests that these sarcoma cell lines were derived from a single clone. It is possible that clonal selection occurred during in vivo tumour evolution or during in vitro passaging to deplete wild-type stroma. Indeed, one sarcoma (2981) harboured a single base pair insertion in the targeted Trp53 locus that was identified in 30 clones sequenced by Sanger sequencing, although deep sequencing of the original tumour identified this single nucleotide insertion in less than 1% of the reads (Supplementary Table 2).
In addition, a single base pair deletion in the targeted Trp53 locus in another sarcoma (2995) detected by deep sequencing was rarely found by Sanger sequencing of the cell lines derived from the same tumour (Fig. 3a,b). The low frequencies of two indels in Trp53 found by Sanger sequencing suggests that it may have developed from independent clones (Fig. 3a). It is also possible that tetraploidization before mutagenesis may account for the detection of more than two distinct indels. In this scenario, however, the frequency of indels generated in a tetraploid intermediate may be more evenly distributed than what we observed. Therefore, it is likely to be that the sarcoma sample may have contained a dominant clone and other rare clones. These data also suggest that the endogenous genetic barcodes generated by Cas9 can be used to study tumour clonality at different stages of tumour development. Here we demonstrate the feasibility of this approach using four genomic loci as potential endogenous barcodes. Multiplexed sgRNAs targeting more than four genomic loci will enable greater sensitivity for identifying and tracking truly clonal populations.
We also performed deep sequencing of the top ten predicted off-target sites in five primary sarcomas in KC mice following Ad-P-Cre injection. The rate of small indels in tumours was similar to the control normal muscle (Supplementary Table 3), indicating no off-target activity at these sites. Sanger sequencing of the on-target sites in exon 7 of Trp53 revealed the presence of deletions up to 281 bp. This class of indel was most commonly represented in our Sanger sequencing data (Fig. 3a). For indel classes that could be detected by deep sequencing, such as the insertion in mouse 2981 and deletion in mouse 2925, the deep sequencing was validated by Sanger sequencing. However, next-generation sequencing did not detect a class of indel, which were large deletions. For example, we found deletions up to 281 bp in size via Sanger sequencing, but these large deletions were not detected by deep sequencing of the same tumour DNA (Fig. 3a,b). Failure of targeted deep sequencing to capture these large deletions class of indels may be due to a limitation of the deep sequencing primer design, which did not adequately flank the targeted loci to allow DNA amplification in the event of a large deletion resulting from DNA repair. Therefore, to capture these larger deletions we searched for deletions in the single exonic off-target site (Celsr1) using Sanger sequencing (Supplementary Table 4). These data showed exon 2 of Celsr1 contained variants, but these were identified as SNPs outside of the Cas9 recognition site (Supplementary Fig. 3). In addition, we performed Surveyor analysis and Sanger sequencing on the remaining off-target sites. No DNA mismatches were detected in those sites, except in off-target site 4, which we also determined to be SNPs (Supplementary Fig. 4).
Other groups have utilized lentiviral delivery for somatic genome editing with the CRISPR-Cas9 system to generate primary cancers of the lung, pancreas and breast16,18,19. We used an adenoviral delivery system, because sarcoma induction via lentiviral expression of Cre in immunocompetent mice is inefficient apparently because of immunosurveillance45. Compared with intramuscular injection of an Ad-Cre into KP mice, we found that intramuscular injection of adenovirus expressing Cre and sgTrp53 into KC mice led to soft tissue sarcoma formation with similar penetrance, time to tumour onset, histologic appearance, mutational load and CNV. In the interest of further simplifying the generation of sarcomas in KC mice, we also performed intramuscular injection of a naked plasmid expressing Cre and sgTrp53. Despite being convenient and economical, intramuscular injection of naked plasmid DNA resulted in primary sarcoma formation with delayed kinetics in only ∼25% of mice. However, the efficiency of naked plasmid delivery to the tumour initiating cell(s) can be increased using IVE46,47, which we hypothesized would increase the penetrance of sarcoma development48. Therefore, we delivered pX333-P-Cre plasmid via IVE and successfully generated tumours in 80% of KC mice (median 10.8 weeks). In KC mice infected with Ad-P-Cre, sarcomas arose over a similar time frame ranging from 7.3–15.7 weeks (median 9.6). Remarkably, we noticed that delivery of pX333-P-Cre plasmid via IVE in mice that constitutively express Cas9 (K-loxP-C mice) resulted in 100% tumour penetrance (median 7.4 weeks) with a more restricted period for tumour formation. This temporal difference between the models may be due to either a cell autonomous or non-cell autonomous mechanism. For example, the endogenous Cas9 expressed in K-loxP-C mice may increase the target population of cells that undergo both Cre-mediated recombination and Cas9-mediated genome editing that are required for tumorigenesis. Alternatively, the endogenous Cas9 expressed in K-loxP-C mice before tumour initiation may delete self-reactive T-cells that recognize Cas9 during normal mouse development. Therefore, in comparison to the KC mice where Cas9 is expressed only after Cre-mediated recombination to generate a potential neoantigen for immune surveillance, the constitutive expression of Cas9 may limit immune surveillance in the K-loxP-C model. Regardless of the mechanism, these data establish in vivo plasmid DNA electroporation as an efficient and facile method of generating sarcomas via CRISPR-Cas9 technology.
We also successfully generated a sarcoma model of MPNST using CRISPR-Cas9 mutagenesis to knockout Nf1 and Trp53 in wild-type mice. Generating this MPNST model only required the time to generate the virus (8 weeks), which is much faster than targeting alleles in ES cells, generating mice with the targeted alleles, crossing the multiple mutant alleles together, and genotyping the mice to generate the desired experimental mice. More importantly, this approach to generating MPNSTs and other sarcomas in wild-type mice gives great flexibility for studying the function of additional genetic mutations without the extra time, effort and expense required for additional crosses.
The mutational landscapes of GEMMs of lung adenocarcinoma were recently reported to have >10-fold lower mutational loads (0.02–0.14 MB−1) compared with human lung cancer from non-smokers (1.97 MB−1)49. We performed whole exome sequencing to profile the mutational load and CNV in the KP primary sarcoma models initiated by Cre alone or Cre and CRISPR-Cas9. We found the mutational load in Cre-mediated KP tumours (0.09 MB−1) was comparable to that of CRISPR-mediated KP tumours (0.07 MB−1) (Fig. 6c). Moreover, the CNV frequency was similar between sarcomas generated with Cre-only (0.05 MB−1) or Cre and CRISPR-Cas9 (0.05 MB−1) (Fig. 6d). Interestingly, the mutational load in the CRISPR-mediated (0.07 MB−1) and Cre-mediated KP sarcomas (0.09 MB−1) was similar to the mutational load in KP lung adenocarcinomas (0.07 MB−1)49. These mutational profiles further demonstrate the similarity between Cre-initiated and CRISPR-Cas9-initiated sarcomas on a genomic level and provide additional evidence beyond the sequencing of potential off-target sites that sarcomas generated by CRISPR-Cas9 technology do not harbour a significant increase in mutations.
In conclusion, we have generated and characterized a combined Cre-loxP, CRISPR-Cas9 model of primary UPS. These sarcomas are comparable to conventional GEMMs of cancer with similar histology, growth kinetics and mutational profiles (Fig. 6). Furthermore, we showed that the CRISPR-Cas9 system can be used to generate autochthonous MPNSTs in wild-type mice. As these models are less expensive and time consuming than traditional GEMMs of sarcoma, they should enable a larger number of investigators to employ primary mouse models for cancer research.
Methods
Mice
KrasLSL-G12D/+ mice were provided by T. Jacks (Massachusetts Institute of Technology). Rosa26LSL-Cas9-EGFP/+ mice were provided by F. Zhang (Massachusetts Institute of Technology). 129/SvJ wild-type mice were purchased from the Jackson Laboratory. Rosa26loxP-Cas9-EGFP/+ mice were generated by crossing Rosa26LSL-Cas9-EGFP/+ mice with Meox2Cre mice and then crossing with C57BL/6J wild-type mice. For the allograft study, male athymic nude (nu/nu) mice (5 to 6 weeks old) were purchased from Taconic Biosciences and maintained in Duke University’s accredited animal facility. For all other experiments, both male and female mice were used. MEFs transduced with 1 × 106 virus particles or transiently transfected with plasmid DNA were injected into the hind limb of the nude mice. Virus was prepared by mixing 25 μl of Ad-sgTrp53-cre (6 × 107 pfu μl−1) or Ad-CMV-EGFP (4 × 107 pfu μl−1) with 600 μl minimal essential medium (Sigma-Aldrich, M4655) or mixing 125 μl of Ad-sgNf1-sgTrp53-Cas9 (4 × 106 pfu μl−1) with 500 μl minimal essential medium. Three microlitres of 2 M CaCl2 was added to each virus prep, mixed and incubated for 15 min at room temperature before injection into either the skeletal muscle (50 μl) or the sciatic nerve (25 μl) after the nerve was exposed by an incision in the surrounding muscle3,25. All animal studies were performed in accordance with protocols approved by the Duke University Institutional Animal Care and Use Committee.
MEF isolation
MEFs were generated from E13.5 to E14.5 embryos following standard procedures. Passage 2–5 MEFs were infected with adenovirus using the stated multiplicity of infection or transiently transfected with plasmids using TransIT-2020 reagent (Mirus Bio LLC, MIR5400) according to the manufacturer’s instructions.
Cell culture
NIH-3T3 cell lines were purchased from ATCC (CRL-1658) and cultured in DMEM medium (Thermo Fisher Scientific, 11965092) supplemented with 10% fetal bovine serum and 1% antibiotic–antimycotic (Thermo Fisher Scientific, 10091148), and incubated at 37 °C with 5% CO2 in a humidified cell-culture incubator. Tumour cells were isolated and expanded in vitro from harvested tumours using standard methods43. Briefly, tumour tissues were minced in the cell-culture hood and then they were digested by dissociation buffer in 1 × PBS (Thermo Fisher Scientific, 14040133) containing collagenase type IV (5 mg ml−1, Thermo Fisher Scientific, 17104-019), dispase (1.3 mg ml−1, Thermo Fisher Scientific, 17105-041) as well as trypsin (0.05%, Thermo Fisher Scientific, 25200056) for about 1 h at 37 °C. Cells were washed with 1 × PBS (Thermo Fisher Scientific, 10010023) and filtered using a 40 μm sieve (Corning, 431750), and cultured for at least four passages before being used for experiments.
Plasmids and adenoviral vectors
The Ad-sgTrp53-Cre adenoviral vector was constructed as follows. The pX333 vector was kindly provided by A. Ventura (Memorial Sloan Kettering Cancer Center). The Cas9 gene in pX333 was replaced by Cre recombinase (Addgene 60229) using AgeI and EcoRI sites by standard cloning methods. For cloning pX333-sgTrp53-Cre, the pX333-Cre vector was digested with BsaI enzyme and ligated to annealed sgRNA oligonucleotides (Supplementary Table 5) targeting mouse Trp53 exon 7. In the final step, pX333-sgTrp53-Cre vector was cloned into the Ad5 adenoviral shuttle vector, which was kindly provided by A. Ventura (Memorial Sloan Kettering Cancer Center) using XhoI and EcoRI sites. The pcDNA3-EGFP vector was purchased from Addgene (13031). Restriction enzymes and T4 ligase were purchased from New England Biolabs. The pX333-sgNF1-sgTrp53-Cas9 adenoviral vector was constructed using a similar approach. Recombinant adenoviruses were generated by Viraquest Inc.
Molecular analysis of recombination
Recombination of the mutant Kras allele and the Cas9 allele were assessed by PCR using AccuPrime Taq HiFi DNA polymerase (Thermo Fisher Scientific, 12346-086)10,50 and utilized primers in Supplementary Table 5.
Tumour analysis
Harvested tissues were fixed in 4% formalin and paraffin-embedded. Immunohistochemistry was performed on 5 μm sections with the ABC kit (Vector Laboratories, PK-7200) with antibodies to S100 (Dako, GA50461-2), MyoD (Dako, M351201-2) and myogenin (Dako, IR06761-2). Sections were developed with 3,3′-diaminobenzidine and counterstained with haematoxylin (Sigma-Aldrich, H3136). Haematoxylin and eosin staining was performed using standard methods. All tissue sections were examined by a sarcoma pathologist (D.M. Cardona).
Frozen tissue processing
The gastrocnemius muscle was harvested from sacrificed mice and immersed in a 4% paraformaldehyde/PBS (Affymetrix, 19943) solution overnight at 4 °C. The tissue was washed in PBS twice for 5 min before immersing in a 30% sucrose/PBS solution overnight at 4 °C. The tissue was then blotted and placed into Tissue-Tek OCT (Sakura Finetek USA, Inc., 4583) compound in embedding molds before snap-freezing in a dry ice and 70% ethanol slurry. Tissue blocks were stored at −80 °C until sectioned at 10 μm using a cryostat. Slides were washed once in PBS, mounted with ProLong Diamond Antifade Mountant with DAPI (Thermo Fisher Scientific, P36962) and imaged using fluorescence microscopy. GFP-positive cells were counted by ImageJ software.
Genomic DNA isolation and indel analysis
Genomic DNA was isolated from both cells and tissues using DNeasy Blood & Tissue Kit (Qiagen, 69504) following the manufacturer’s protocol. PCR amplification of mouse Trp53 targeted exon 7 was performed with specific primers using AccuPrime Taq HiFi DNA polymerase. PCR amplification of mouse Nf1 targeted exon 7 was performed with specific primers. PCR reaction was subjected to a re-annealing process to enable heteroduplex formation in a thermocycler37. CRISPR-Cas9-induced indels were detected using Surveyor Mutation Detection Kits (IDT, 706021) following the recommended protocol. Homoduplex and cleaved heteroduplex products were visualized with ultraviolet illumination after gel electrophoresis in 2% Tris-borate-EDTA agarose gels, or 4–20% Tris-borate-EDTA polyacrylamide gels.
Molecular analysis of KrasG12D mutation
PCR amplification of mouse wild-type Kras allele and the KrasG12D mutant allele was performed with specific primers (Supplementary Table 1). The PCR product was either used directly or cloned into the pCR4-TOPO TA vector (Thermo Fisher Scientific, K457501) for Sanger sequencing. A total of five clones from each vector were Sanger sequenced.
Western blot analysis of p53 and Nf1 knockout
NIH-3T3, KP tumour and MPNST tumour cell lines were treated with 0.5 μg ml−1 doxorubicin (Sigma-Aldrich, D1515-10MG) for 8 h before sample harvest. Samples were lysed in RIPA buffer for 30 min on ice (Sigma-Aldrich, R0278) then centrifuged at 10,000 g for 20 min at 4 °C. Lysate supernatant was separated from debris into a new tube and protein concentration was determined by BCA assay (Pierce, 23225). The lysate was boiled in 4 × sample buffer (Thermo Fisher Scientific, 84788) at 95 °C for 5 min, then cooled to room temperature before loading in a 3–8% Tris-Acetate protein gel (Thermo Fisher Scientific, EA03785BOX). Samples were electrophoresed at 150 V for 80 min before transfer to nitrocellulose. Total protein was determined using REVERT total protein stain (Li-Cor Biosciences, P/N 926-11011) and an Odyssey CLx (Li-Cor Biosciences, 9194), then washed and destained before blocking in 5% non-fat dry milk in tris-buffered saline (TBS, Corning, 46-012-CM). Next, membranes were incubated overnight at 4 °C with primary antibodies diluted in TBS-T (0.1% Tween-20): p53, 1:250 dilution (1C12, Cell Signaling Technology); Nf1, 1:500 dilution (A300-A140M, Bethyl Laboratories). Membranes were washed three times in TBS-T for 5 min before secondary antibody incubation with goat anti-rabbit IRDye800 (Li-Cor Biosciences, P/N 925-32211) and goat anti-mouse IRDye680 (Li-Cor Biosciences, P/N 925-68070) both at 1:10,000 dilutions in TBS-T for 1 h at room temperature. The membranes were washed three times in TBS-T for 5 min and imaged using an Odyssey CLx (Li-Cor Biosciences). Image analysis for normalization and quantification were performed using Image Studio (Version 5.2, Li-Cor Biosciences, P/N 9140-500). Full blots with protein ladders (Li-Cor Biosciences, P/N 928-60000) are shown in Supplementary Fig. 7.
Clonality study
Cells were disassociated from four independent sarcomas and passaged at least five times. Genomic DNA was isolated from each tumour cell line. Trp53 exon 7 was amplified by PCR and cloned into the pCR4-TOPO TA vector (Thermo Fisher Scientific). Thirty clones from each vector were Sanger sequenced. On-target chromosome 17 site was amplified by PCR using genomic DNA isolated from each tumour cell line and cloned into pCR4-TOPO TA vector. Ten clones from each vector were Sanger sequenced for the on-target chromosome 17 site.
Whole exome sequencing
Genomic DNA was sheared to 250 bp using the Covaris S2 platform. To increase the throughput of exome library preparation, a custom 96-well barcode system was designed based on the Illumina sequencing system, whereby a separate sequencing read is used to identify the barcode of individual sequencing libraries. Custom 8-mer barcodes compatible with the 3-read Illumina Hiseq v2 platform were designed enforcing an edit distance of ≥3 to greatly minimize the possibility of sample cross-contamination during sequencing. Additional measures were applied to ensure optimal base diversity at each sequencing cycle.
Oligonucleotides were ordered with standard Illumina barcode modifications of 5′-phosphorylation, a phosphorothioate bond between the last two bases on the 3′-end, and HPLC purification. Oligonucleotides were annealed by standard protocols and diluted to a 15 M working concentration in elution buffer (EB). Pre-capture libraries were prepared with standard library preparation protocols using the KAPA Hyper kit (Kapa Biosystems) and then pooled at equal volume and subjected to exome-capture using the Agilent Mouse All Exon kits, followed by high-throughput sequencing on the Illumina Hiseq 2500 platform.
Reads in fastq format were pre-processed with GATK51 version 3.2 to remove Illumina adapter sequences and phred-scaled base qualities of ten and below. After GATK processing, reads were mapped to mm10 using Burrows-Wheeler Aligner52 version 0.7.7 with the mem algorithm. Reads were sorted with Novoalign V2.08.03 novosort. PCR/optical duplicates were marked by Picard. Base-quality recalibration and localized indel realignment was performed using GATK53.
Joint variant calling was performed to identify single nucleotide variant and indels using GATK Haplotype Caller53 by first assembling plausible haplotypes to reduce alignment errors and to improve the accuracy of variant calling. Somatic variants were identified using MuTect54 by comparing tumours with matched normal. Variants were annotated using SnpEff55.
Variant filtering was performed by first taking the intersection of the variants from both the variant callers (MuTect and Haplotype caller) to identify high-quality somatic variants. Additional filtering was performed to retain variants with good coverage (≥15 reads), allele frequency (≥0.2), exonic variants predicted to change the amino acid sequence (missense, frameshift, stop gain and splice site variants). Mutation load per MB was calculated by dividing the count of the filtered somatic variants by the size of the exome target region (49.5 MB).
PCR amplification of each somatic variant was performed with specific primers using AccuPrime Taq HiFi DNA polymerase (Thermo Fisher Scientific). The PCR product was either used directly or cloned into the pCR4-TOPO TA vector (Thermo Fisher Scientific) for Sanger sequencing. A total of 10 or 30 clones from each vector were Sanger sequenced. Total 38 somatic mutation calls were validated independently, which implied 84.2% accuracy of the calling method. Validated mutations for each tumour tissue are listed in Supplementary Table 6.
Deep sequencing
The sgTrp53 target and the top ten candidate off-target sites were examined in five sarcomas generated by Ad-P-Cre in KC mice by deep sequencing following a protocol described previously11. PCR of genomic DNA from 5 sarcomas and the normal muscle from the same mouse was carried out using primers spanning the on-target Trp53 region, on-target chromosome 17 site and 10 off-target sites (Supplementary Tables 2 and 3). PCR was carried out to add Illumina flowcell binding sequences and experiment-specific barcodes on the 5′-end of the primer sequence (Supplementary Table 5). All PCR products were pooled together and sequenced on an Illumina MiSeq instrument. Indel analysis was performed as previously described using default CRISPRresso settings and a 20 bp window11,56. Briefly, 3′-ends of each paired-end reads are overlapped, because PCR products were <250 bp in length. This overlap was applied to generate a consensus PCR amplicon for each paired-end read by single ungapped alignment (parameterized to score each match as 5 and each mismatch as −4). Trimmed fragments were aligned to the reference genome. Then, each of the aligning fragments was aligned to the reference genome sequence of the PCR product with a global affine alignment with the following parameterization: match=5, mismatch=−4, gap open=−5 and gap exten=−2. If the fragment did not have greater than 60% identity with original expected PCR amplicon or it had a top-scoring BLAT alignment that was different than the expected PCR product, this fragment was discard from downstream analysis. Alignments were trimmed to a 20 bp window centred three base pairs 5′ of the protospacer-adjacent motif (PAM), the predicted site of Cas9 nuclease activity. Next, indel statistics were further gathered from windows separately for each sample by counting gaps in the query and subject sequences of the resulting truncated alignments and tabulating numbers of fragments having any indels in the windows.
In vivo electroporation
After mice were anaesthetized, 50 μg of naked DNA plasmid diluted in sterile saline was injected into the gastrocnemius using a 31-gauge insulin syringe. A pair of needle electrodes with a 5 mm gap was inserted into the muscle to encompass the DNA injection site, and electric pulses were delivered using an electric pulse generator (Electro Square Porator ECM830; BTX, San Diego, CA). Three 100 V pulses followed by three additional 100 V pulses of the opposite polarity were administered to each injection site at a rate of 1 pulse per 50 ms with each pulse being 200 ms in duration.
Statistical analyses
Results are presented as means ±s.e.m. unless otherwise indicated. Before analysis, all data were displayed graphically to determine whether parametric or non-parametric tests should be used. Two-tailed Student’s t-test was performed to compare the means of two groups. For tumour kinetic studies, Kaplan-Meier analysis was performed with the log-rank test for statistical significance. Significance was assumed at P<0.05. All calculations were performed using Prism 6 (GraphPad).
Data availability
All data generated or analysed during this study are included in this published article and its Supplementary Information files. The data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. All sequencing data are deposited at the European Genome-phenome Archive with accession number: EGAS00001002459.
Additional information
How to cite this article: Huang, J. et al. Generation and comparison of CRISPR-Cas9 and Cre-mediated genetically engineered mouse models of sarcoma. Nat. Commun. 8, 15999 doi: 10.1038/ncomms15999 (2017).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Hanahan, D., Wagner, E. F. & Palmiter, R. D. The origins of oncomice: a history of the first transgenic mice genetically engineered to develop cancer. Genes Dev. 21, 2258–2270 (2007).
Politi, K. & Pao, W. How genetically engineered mouse tumor models provide insights into human cancers. J. Clin. Oncol. 29, 2273–2281 (2011).
Kirsch, D. G. et al. A spatially and temporally restricted mouse model of soft tissue sarcoma. Nat. Med. 13, 992–997 (2007).
Moding, E. J. et al. Atm deletion with dual recombinase technology preferentially radiosensitizes tumor endothelium. J. Clin. Invest. 124, 3325–3338 (2014).
Moding, E. J. et al. Tumor cells, but not endothelial cells, mediate eradication of primary sarcomas by stereotactic body radiation therapy. Sci. Transl. Med. 7, 278ra34 (2015).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Ran, F. A. et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 520, 186–191 (2015).
Platt, R. J. et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell 159, 440–455 (2014).
Nelson, C. E. et al. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 351, 403–407 (2016).
Carroll, K. J. et al. A mouse model for adult cardiac-specific gene deletion with CRISPR/Cas9. Proc. Natl Acad. Sci. USA 113, 338–343 (2016).
Maddalo, D. et al. In vivo engineering of oncogenic chromosomal rearrangements with the CRISPR/Cas9 system. Nature 516, 423–427 (2014).
Heckl, D. et al. Generation of mouse models of myeloid malignancy with combinatorial genetic lesions using CRISPR-Cas9 genome editing. Nat. Biotechnol. 32, 941–946 (2014).
Weber, J. et al. CRISPR/Cas9 somatic multiplex-mutagenesis for high-throughput functional cancer genomics in mice. Proc. Natl Acad. Sci. USA 112, 13982–13987 (2015).
Sánchez-Rivera, F. J. et al. Rapid modelling of cooperating genetic events in cancer through somatic genome editing. Nature 516, 428–431 (2014).
Xue, W. et al. CRISPR-mediated direct mutation of cancer genes in the mouse liver. Nature 514, 380–384 (2014).
Chiou, S.-H. et al. Pancreatic cancer modeling using retrograde viral vector delivery and in vivo CRISPR/Cas9-mediated somatic genome editing. Genes Dev. 29, 1576–1585 (2015).
Annunziato, S. et al. Modeling invasive lobular breast carcinoma by CRISPR/Cas9-mediated somatic genome editing of the mammary gland. Genes Dev. 30, 1470–1480 (2016).
Rodriguez, R., Rubio, R. & Menendez, P. Modeling sarcomagenesis using multipotent mesenchymal stem cells. Cell Res. 22, 62–77 (2012).
Blum, J. M. et al. Distinct and overlapping sarcoma subtypes initiated from muscle stem and progenitor cells. Cell Rep. 5, 933–940 (2013).
Hatley, M. E. et al. A mouse model of rhabdomyosarcoma originating from the adipocyte lineage. Cancer Cell 22, 536–546 (2012).
Minas, T. Z. et al. Combined experience of six independent laboratories attempting to create an Ewing sarcoma mouse model. Oncotarget 8, 34141–34163 (2016).
Post, S. M. Mouse models of sarcomas: critical tools in our understanding of the pathobiology. Clin. Sarcoma Res. 2, 20 (2012).
Dodd, R. D. et al. NF1 deletion generates multiple subtypes of soft-tissue sarcoma that respond to MEK inhibition. Mol. Cancer Ther. 12, 1906–1917 (2013).
Jackson, E. L. et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 15, 3243–3248 (2001).
Attardi, L. D., de Vries, A. & Jacks, T. Activation of the p53-dependent G1 checkpoint response in mouse embryo fibroblasts depends on the specific DNA damage inducer. Oncogene 23, 973–980 (2004).
Hegi, M. E. et al. Allelotype analysis of mouse lung carcinomas reveals frequent allelic losses on chromosome 4 and an association between allelic imbalances on chromosome 6 and K-ras activation. Cancer Res. 54, 6257–6264 (1994).
Kerr, E. M., Gaude, E., Turrell, F. K., Frezza, C. & Martins, C. P. Mutant Kras copy number defines metabolic reprogramming and therapeutic susceptibilities. Nature 531, 110–113 (2016).
Zhang, Z. et al. Wildtype Kras2 can inhibit lung carcinogenesis in mice. Nat. Genet. 29, 25–33 (2001).
Mito, J. K. et al. Cross species genomic analysis identifies a mouse model as undifferentiated pleomorphic sarcoma/malignant fibrous histiocytoma. PLoS ONE 4, e8075 (2009).
Levine, D. S., Sanchez, C. A., Rabinovitch, P. S. & Reid, B. J. Formation of the tetraploid intermediate is associated with the development of cells with more than four centrioles in the elastase-simian virus 40 tumor antigen transgenic mouse model of pancreatic cancer. Proc. Natl Acad. Sci. USA 88, 6427–6431 (1991).
Margolis, R. L., Lohez, O. D. & Andreassen, P. R. G1 tetraploidy checkpoint and the suppression of tumorigenesis. J. Cell. Biochem. 88, 673–683 (2003).
Fujiwara, T. et al. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature 437, 1043–1047 (2005).
Cho, S. W. et al. Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. Genome Res. 24, 132–141 (2014).
Fu, Y. et al. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat. Biotechnol. 31, 822–826 (2013).
Ran, F. A. et al. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Lee, W. et al. PRC2 is recurrently inactivated through EED or SUZ12 loss in malignant peripheral nerve sheath tumors. Nat. Genet. 46, 1227–1232 (2014).
Cichowski, K. et al. Mouse models of tumor development in neurofibromatosis type 1. Science 286, 2172–2176 (1999).
Whitley, M. J. et al. A mouse-human phase 1 co-clinical trial of a protease-activated fluorescent probe for imaging cancer. Sci. Transl. Med. 8, 320ra4 (2016).
Kim, S. et al. Efficacy of phosphatidylinositol-3 kinase inhibitors in a primary mouse model of undifferentiated pleomorphic sarcoma. Sarcoma 2012, 680708 (2012).
Mito, J. K. et al. Oncogene-dependent control of miRNA biogenesis and metastatic progression in a model of undifferentiated pleomorphic sarcoma. J. Pathol. 229, 132–140 (2013).
Sachdeva, M. et al. MicroRNA-182 drives metastasis of primary sarcomas by targeting multiple genes. J. Clin. Invest. 124, 4305–4319 (2014).
van Overbeek, M. et al. DNA repair profiling reveals nonrandom outcomes at Cas9-mediated breaks. Mol. Cell 63, 633–646 (2016).
DuPage, M., Mazumdar, C., Schmidt, L. M., Cheung, A. F. & Jacks, T. Expression of tumour-specific antigens underlies cancer immunoediting. Nature 482, 405–409 (2012).
Aihara, H. & Miyazaki, J. Gene transfer into muscle by electroporation in vivo. Nat. Biotechnol. 16, 867–870 (1998).
Maresch, R. et al. Multiplexed pancreatic genome engineering and cancer induction by transfection-based CRISPR/Cas9 delivery in mice. Nat. Commun. 7, 10770 (2016).
Tsumura, H., Yoshida, T., Saito, H., Imanaka-Yoshida, K. & Suzuki, N. Cooperation of oncogenic K-ras and p53 deficiency in pleomorphic rhabdomyosarcoma development in adult mice. Oncogene 25, 7673–7679 (2006).
McFadden, D. G. et al. Mutational landscape of EGFR-, MYC-, and Kras-driven genetically engineered mouse models of lung adenocarcinoma. Proc. Natl Acade. Sci. USA 113, E6409–E6417 (2016).
Jackson, E. L. et al. The differential effects of mutant p53 alleles on advanced murine lung cancer. Cancer Res. 65, 10280–10288 (2005).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Li, H. & Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26, 589–595 (2010).
Van der Auwera, G. A. et al. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinformatics 43, 11.10.1–33 (2013).
Cibulskis, K. et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat. Biotechnol. 31, 213–219 (2013).
Cingolani, P. et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 6, 80–92 (2012).
Pinello, L. et al. Analyzing CRISPR genome-editing experiments with CRISPResso. Nat. Biotechnol. 34, 695–697 (2016).
Acknowledgements
We thank David Ousterout for suggestions on designing the sgRNA to Trp53. We also thank Andrea Ventura and Danilo Maddalo for sharing plasmids and helpful advice to generate vectors to utilize the CRISPR-Cas9 system. This work was supported by the National Cancer Institute of the US NIH under award numbers F30CA206424 (M.C.), T32GM007171 (M.C. and M.J.W.) and R35CA197616 (D.G.K.), and by the Department of Defense under award number W81XWH-14-1-0067 (D.G.K.).
Author information
Authors and Affiliations
Contributions
J.H., M.C., M.J.W. and D.G.K. designed experiments. J.H., M.C., M.J.W., H.-C.K., E.S.X., A.W., L.L., Y.M., Y.M.M., D.V.M., O.M.L., J.N.R.-H. and R.D.D. performed experiments. J.H., M.C. and E.S.X. performed IVE. W.C.E. and R.D.D. performed sciatic nerve injections. M.C., Y.M. and R.D.D. performed histology and D.M.C. analysed all IHC sections. A.R. and S.S.D. performed and analysed whole exome sequencing. J.H., M.C., H.-C.K. and C.E.N. performed and analysed deep sequencing. C.A.G. and D.G.K. provided critical advice for the manuscript. J.H., M.C., M.J.W. and D.G.K. drafted the first version of the manuscript. All authors edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
C.A.G. is a founder and scientific advisor to Element Genomics. The remaining authors declare no competing financial interests.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Huang, J., Chen, M., Whitley, M. et al. Generation and comparison of CRISPR-Cas9 and Cre-mediated genetically engineered mouse models of sarcoma. Nat Commun 8, 15999 (2017). https://doi.org/10.1038/ncomms15999
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms15999
This article is cited by
-
Precise genome-editing in human diseases: mechanisms, strategies and applications
Signal Transduction and Targeted Therapy (2024)
-
Discrepancies in indel software resolution with somatic CRISPR/Cas9 tumorigenesis models
Scientific Reports (2023)
-
Utility of CD138/syndecan-1 immunohistochemistry for localization of plasmacytes is tissue-dependent in B6 mice
BMC Research Notes (2022)
-
Current applications and future perspective of CRISPR/Cas9 gene editing in cancer
Molecular Cancer (2022)
-
Malignant peripheral nerve sheath tumor: models, biology, and translation
Oncogene (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.