Abstract
The serine/threonine kinase LKB1 regulates various cellular processes such as cell proliferation, energy homeostasis and cell polarity and is frequently downregulated in various tumours. Many downstream pathways controlled by LKB1 have been described but little is known about the upstream regulatory mechanisms. Here we show that targeting of the kinase to the membrane by a direct binding of LKB1 to phosphatidic acid is essential to fully activate its kinase activity. Consequently, LKB1 mutants that are deficient for membrane binding fail to activate the downstream target AMPK to control mTOR signalling. Furthermore, the in vivo function of LKB1 during development of Drosophila depends on its capacity to associate with membranes. Strikingly, we find LKB1 to be downregulated in malignant melanoma, which exhibit aberrant activation of Akt and overexpress phosphatidic acid generating Phospholipase D. These results provide evidence for a fundamental mechanism of LKB1 activation and its implication in vivo and during carcinogenesis.
Similar content being viewed by others
Introduction
The serine/threonine kinase LKB1 is ubiquitously expressed and highly conserved throughout evolution. It has been demonstrated to function as a ‘master kinase’ potentially activating several downstream kinases1. Apart from its implication in carcinogenesis LKB1 plays a role in various cellular signalling pathways such as Wnt-, TGFβ-signalling or the mTOR-pathway reviewed by Vaahtomeri and Makela2. The latter one is controlled by LKB1-mediated activation of AMP-dependent kinase (AMPK), which is essential for cell survival and polarity in Drosophila and vertebrates, in particular under energetic stress3,4,5,6,7. Mechanistically, AMPK phosphorylates (among others) Raptor (a core component of mTOR complex 1) and TSC2 (an mTOR inhibitor), resulting in reduced mTOR activity8,9,10,11,12,13,14,15. Consequently, the LKB1-AMPK-axis is believed to be a key modulator in carcinogenesis and cell polarity16.
Apart from its function in cell proliferation and tumour suppression, LKB1 is directly implicated in the establishment and maintenance of cell polarity in different cell types and organisms17.
Although many downstream pathways mediating the function of LKB1 have been described, little is known about the upstream mechanisms regulating LKB1 activity. Two pseudokinases tightly control the localization and kinase activity of LKB1: STRADα (Ste20-like kinase (Stlk) in Drosophila) and Mo25 form a stable ternary complex with LKB1 (ref. 18). Both proteins enhance the export of LKB1 from the nucleus into the cytoplasm and increase its kinase activity18,19. Secondly, the conserved C-terminus of LKB1 is farnesylated in vivo and thereby might directly interact with the plasma membrane to attach the protein to the cell cortex. Although the majority of the protein accumulates at the plasma membrane of polarized (epithelial) cells, farnesylation has been reported to be not essential for the (tumour suppressor) function of LKB1 in mammalian cells or mice but might be essential for oogenesis in Drosophila 20,21,22. Therefore the question remains, whether membrane association of LKB1 is essential for the kinase activity and function of the protein during development and tumour suppression.
Here we report that binding of LKB1 to membranes by direct interaction with phospholipids, in particular to phosphatidic acid, is essential for its function in vivo during development of Drosophila. Membrane association of LKB1 is required for its kinase activity and for efficient activation of AMPK in cultured mammalian cells, thus contributing to the tumour suppressor function of LKB1. Strikingly, we reveal a strong correlation between overexpression of Phospholipase D (PLD), which increases cellular levels of phosphatidic acid, downregulation of LKB1 and enhanced activity of mTOR in malignant melanoma, thus likely contributing to the pathogenesis of malignant melanoma.
Results
LKB1 localizes to the cortex of epithelial cells and neuroblasts
The activity of kinases can be modulated for instance by directly influencing their enzymatic activity (e.g., by a conformational change via phosphorylation) or by targeting the protein to different subcellular compartments. In cultured mammalian cells, LKB1 accumulates in the nucleus in many cell lines and only a minor fraction of the protein is found in the cytoplasm or at the cytocortex23,24,25. LKB1 has been demonstrated to shuttle from the cytoplasm into the nucleus and back, with its two co-factors, STRADα and Mo25 enhancing cytoplasmic localization and activating the kinase18,19,26.
Whereas endogenous LKB1 accumulates predominately in the nucleus of non-transformed fibroblasts (Fig. 1a), epithelial cells (Madin Darby Canine Kidney, MDCK) exhibit a staining of endogenous LKB1 exclusively at the cell-cell contacts, partly overlapping with the AJ- and TJ markers E-Cadherin and ZO-1 (Fig. 1b and ref. 27). Similar, endogenous LKB1 localizes to the (lateral) membrane in epithelial cells in vivo (colon or salivary glands) (Fig. 1c,d). Remarkably, polarized epithelial cells in culture (MDCK) and in situ (colon and salivary gland) do not exhibit nuclear staining of LKB1.
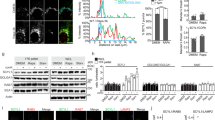
(a) In non-transformed mammalian fibroblasts (IMR90 cells), endogenous LKB1 accumulates mostly in the nucleus. (b) In polarized epithelial cells (MDCK), LKB1 is targeted to the cell-cell contacts, partly colocalizing with E-Cadherin (E-Cad) and Zonula occludens protein 1 (ZO-1). (c,d) Sections of paraffin-embedded colon (c) and salivary gland tissues (d) show a localization of LKB1 at the lateral plasma membrane in situ (arrows). (e–h) LKB1 localizes to the lateral plasma membrane in epithelial cells of the Drosophila embryonic epidermis (e) and to the cortex of neuroblasts (f–h). (i–n) Wild-type GFP-LKB1 as well as farnesylation deficient LKB1 (LKB1C564A) expressed from its endogenous promoter localize correctly to the (lateral) membrane of epithelial cells of the embryonic epidermis (i,j), of the follicular epithelium (m,n) and of neuroblasts (k,l). (o) Lethality tests of LKB1 variants as described in the methods section. Scale bars are 20 μm in a–d, 5 μm in e–n. Experiments were performed in triplicates. Error bars represent s.d. and statistical significance was determined using ANOVA: P<0.0001, ***P<0.01, *P>0.05, not significant (NS).
In Drosophila cells endogenous LKB1 is also not found in the nucleus but localizes at the cortex of female germ line cells as well as at the lateral membrane in epithelial cells22, whereas it shows a diffuse cytoplasmic pattern in neural stem cells of Drosophila larval neuroblasts (NBs)28. Similar, the Caenorhabditis elegans orthologue, PAR-4, regulating asymmetric cell division of the zygote, localizes to the entire cell cortex29. To test which mechanisms target LKB1 to the cortex, we raised an antibody against LKB1 and confirmed that in epithelial cells of the embryonic epidermis, endogenous LKB1 is localized laterally, co-staining with α-spectrin (Fig. 1e), but also overlapping with the zonula adherens (ZA), marked by Drosophila E-Cadherin (DE-Cad) (Fig. 1e). Notably, in embryonic NBs, we detect a clear cortical LKB1 staining in interphase as well as during mitosis (Fig. 1f–h). However, in contrast to key regulators of asymmetric cell division like the apical localized Bazooka (Baz) protein and the basally segregated adaptor protein Miranda (Mir), LKB1 does not show a polarized distribution during mitosis (Fig. 1f–h). Thus, LKB1 localizes predominately to the (lateral) plasma membrane in polarized epithelial cells and NBs in Drosophila and mammals.
Farnesylation of LKB1 is not essential for its localization
The C-terminus of LKB1 can be farnesylated21,30, establishing a putative membrane targeting domain—however, this modification does not seem to be important for its tumour suppressor function in mammals20 and for viability of mice21. In the Drosophila germ line, a block of farnesylation leads to a weaker cortical association and disturbed oocyte polarity22. To test whether this is also true for epithelial cells and NBs, we used the endogenous LKB1 promoter to express a GFP-tagged LKB1 wild-type protein or an LKB1 version with a Cys564-Ala substitution in the fly (farnesylation-deficient LKB1, lkb1::GFP-LKB1C564A). Wild-type GFP-LKB1 is expressed at similar protein level as endogenous LKB1 (Supplementary Fig. 1a) and localizes to the lateral cortex of epithelial cells and the cortical membrane in NBs indistinguishable from the endogenous protein (Fig. 1i,k). Surprisingly, in the embryonic epidermis and embryonic NBs, GFP-LKB1C564A shows the same subcellular localization as its wild-type counterpart (Fig. 1j,l). The farnesylation-deficient protein shows a more cytosolic distribution only in epithelial cells surrounding the oocyte (follicular epithelium) although a substantial fraction of the protein is still associated with the lateral membrane (Fig. 1n compared to wild-type GFP-LKB1 in Fig. 1m).
Notably, LKB1C564A expressed from its endogenous promoter is able to rescue an lkb1-null allele (lkb1 X5) to a large extent (52% surviving flies in comparison to 69% for wild-type LKB1, Fig. 1o), indicating that farnesylation of LKB1 is not essential for the function of the protein in vivo.
LKB1 directly binds to phospholipids
In order to further elucidate the targeting of LKB1 to the plasma membrane, we used Schneider R+ (SR+) cells as they do not exhibit an intrinsic polarity and do not express transmembrane proteins like DE-Cad, Crumbs or Echinoid, qualifying them as a model for the analysis of direct plasma membrane targeting. As expected, GFP-LKB1 localizes to the plasma membrane in transfected S2R+ cells (Fig. 2c). In contrast, the farnesylation motif alone (GFP fused to the last 15 amino acids of LKB1, GFP-LKB1552-C) is not sufficient to target the protein to the cortex (Fig. 2d), which is in line with the hypothesis that stable membrane binding requires protein modifications (e.g., palmitoylation) or membrane binding domains31 in addition to conjugation with farnesyl acid. In contrast, a longer C-terminal fragment of LKB1 (LKB1512-C and LKB1536-C) exhibits a robust cortical localization apart from a nuclear staining (Fig. 2e; Supplementary Fig. 2a). Furthermore, in embryos, a substantial fraction of GFP-LKB1512-C localizes to the lateral membrane (Fig. 2g).
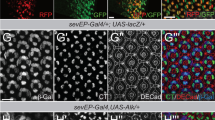
(a) Schematic drawing showing Drosophila LKB1 with basic residues and the farnesylation motif within its C-terminal region highlighted. (b) Lipid overlay assay of recombinant LKB1 and LKB1ΔLB (=LKB1K546A R547A R548A K550A K551A K539A K540A K541A) using diverse lipids spotted on nitrocellulose membrane. (c–f) GFP-LKB1 and GFP-LKB1512-C localize to the cell cortex of transfected S2R cells (c,e), whereas a fusion protein of the last 16aa of LKB1 with GFP (GFP-LKB1552-C) or GFP-LKB1ΔLB does not (d,f). (g–j) GFP-LKB1512-C accumulates at the lateral membrane of epithelial cells in the embryonic epidermis (g), similar to full length GFP-LKB1 (h), whereas GFP-LKB1ΔLB exhibits a cytosolic localization in epithelial cells of the embryonic epidermis (i) and of the follicular epithelium (k) as well as in neuroblasts (j). Scale bars are 5 μm.
Membrane association can be achieved not only by the interaction with transmembrane or membrane associated proteins but also by the direct binding to the lipid bilayer. Several protein domains are known to facilitate protein–lipid interactions. Beside larger domains, polybasic motifs have been described for several proteins to bind to (phospho)-lipids, in particular to phosphatidic acid32. Indeed, a C-terminal polybasic motif (aa 539–551, termed lipid-binding motif=LB, Fig. 2a) facilitates a direct binding of DmLKB1 to PA, PtdIns(5)P, PtdIns(3,4,5)P3 and PtdIns(4,5)P2 as determined by a lipid overlay assay (Fig. 2b). A strong binding to PA (and to some extent to PtdIns(3,4,5)P3 and PtdIns(4,5)P2) was confirmed in liposome flotation assays (Supplementary Fig. 2b). Mutation of this motif (mutation of Arg and Lys in LKB1-LB to Ala, LKB1ΔLB) abolished liposome association in vitro (Supplementary Fig. 2b) as well as membrane association in cultured cells (Fig. 2f) and in Drosophila epithelia and neural stem cells (Fig. 2h–k).
Membrane-bound LKB1 is crucial for Drosophila development
LKB1 has been implicated in various cellular functions, among them cell proliferation control, suppression of tumour growth and regulation of cell polarity in various cell types2,17. To address the question whether membrane targeting of LKB1 is crucial for its physiological function, we performed rescue experiments with GFP-LKB1 expressed from its endogenous promoter. As indicated above, not only wild-type LKB1 but also LKB1C564A can rescue the embryonic lethality of maternal and zygotic mutant flies to a substantial extent, indicating that wild-type and farnesylation-deficient GFP-LKB1 can substitute endogenous LKB1 in all tissues (Fig. 1o). Notably, GFP-DmLKB1ΔLB still exhibits a residual rescue capacity (8% of surviving flies in contrast to 69% for wild-type DmLKB1, Fig. 1o), which might be due to a transient or weak membrane binding mediated by the farnesylation anchor. Indeed, removal of the farnesylation motif in GFP-LKB1ΔLB (GFP-LKB1ΔLB C564A) totally abolishes the rescue capacity of the mutant protein and all mutant flies die during embryonic stages like the null allele lkb1X5 (Fig. 1o). Moreover, lkb1-mutant flies expressing GFP-LKB1ΔLB C564A exhibit the same polarity defects as the null allele alone (e.g., disruption of the anterior–posterior polarity of ovaries, Fig. 3c, compared with wild-type LKB1 rescue in a and lkb1-mutant phenotype in b).
(a–c) Immunostainings of lkb1-mutant ovaries (b) exhibit a disturbed localization of Staufen, which is rescued by expression of GFP-LKB1 (a) but not of GFP-LKB1ΔLB C564A (c). (d) Immunoblotting of LKB1 variants expressed in embryos demonstrating that the protein stability of GFP-LKB1ΔLB C564A is strongly reduced. (e) Overexpression of LKB1, but not of a kinase-dead version of LKB1 (LKB1D317A=LKB1KD) or GFP-LKB1ΔLB C564A results in an impaired eye morphology (‘rough eye’ phenotype). (f,g) In vitro kinase assays of GFP-LKB1/GFP-STRAD purified from transfected S2R cells demonstrate a strong decrease in the kinase activity of LKB1ΔLB C564A measured by autophosphorylation (32P GFP-LKB1) and phosphorylation of recombinant AMPK (32P GST-AMPK108-280) (f). The addition of PA-enriched liposomes (as described in materials and methods) increased the kinase activity of wild type but not of membrane-binding deficient LKB1 (g). Activity of GFP-LKB1 was set as 100%. Inputs were visualized by Western Blot against GFP (GFP, GFP-Stlk and GFP-LKB1) and by Coomassie Brilliant Blue staining (CCB, GST-AMPK108-280) Scale bars are 5 μm in c–k and m, 100 μm in o and p, 10 μm in l and n. Experiments were performed in triplicates. Error bars represent s.d. and statistical significance was determined using ANOVA: P<0.001, ***P<0.01, **P<0.05, not significant (NS).
Western blot analyses of lysates from embryos expressing LKB1 variants from the endogenous promoter reveal that GFP-LKB1ΔLB C564A is unstable or rapidly degraded (Fig. 3d), indicating that the association of LKB1 with membranes is essential for protein stability. In contrast, transient membrane association of LKB1ΔLB, which is probably mediated by the farnesylation anchor, is sufficient for protein stabilization, as no differences in protein levels are detectable between wild-type LKB1 and lipid-binding-deficient LKB1 (Fig. 3d).
However, overexpression of GFP-DmLKB1ΔLB C564A in lkb1-mutant flies using the UAS/GAL4 system instead of the endogenous lkb1-promoter does not result in detectable rescue capacity (Supplementary Fig. 3a). Similar, the overexpression phenotype (rough eye formation) of LKB1 is abolished in GFP-DmLKB1ΔLB C564A similar to a kinase dead version (Fig. 3e). Thus, we assume that it is very unlikely that impaired functionality of GFP-DmLKB1ΔLB C564A is only due to the instability of the protein.
To further substantiate this assumption, we fused two different heterologous membrane-binding domains to the C terminus of GFP-LKB1ΔLB C564A: The PH-domain of human phospholipase Cδ (ref. 33) that preferentially binds to PtdIns(4,5)P2 (resulting in GFP-LKB1ΔLB C564A-PH(PLCδ)), but is also capable of binding to PA with a high affinity34, and the PH-domain of human Akt1, (GFP-LKB1ΔLB C564A-PH(Akt1)) with a high affinity to PtdIns(3,4,5)P3 (ref. 35). Similar to PLCδ, Akt1 has recently been demonstrated to bind PA in vitro 36. Strikingly, both chimeric proteins stabilize the protein expression of GFP-LKB1ΔLB C564A (Fig. 3d), localize at least partly to the lateral plasma membrane (Supplementary Fig. 4a,b) and are capable of rescuing the lkb1 null allele to a large extent (56 and 48% respectively, Fig. 1o). These results confirm our hypothesis that membrane association of LKB1 is crucial for its function in vivo.
To test whether membrane association is essential for the kinase function of LKB1, we performed in vitro kinase assays using the GFP-DmLKB1/STRADα complex purified from transfected S2R cells. Indeed GFP-DmLKB1ΔLB C564A exhibits a strongly decreased kinase activity (46% in autophosphorylation and 30% in AMPK-phosphorylation relative to wild-type GFP-LKB1, Fig. 3f). Furthermore, addition of PA-enriched liposomes to GFP-DmLKB1/STRADα strongly increases the kinase activity of the LKB1 complex (autophosphorylation 1.6-fold and AMPK-phosphorylation 2.4-fold (Fig. 3g).
Reduced kinase activity of LKB1ΔLB C654A is neither due to impaired association with the canonical binding partners STRADα or Mo25, which enhance LKB1 activity, nor due to decreased substrate binding (Supplementary Fig. 3b). Therefore, these data suggest that the lipid binding capacity of DmLKB1 is essential for its kinase function.
The function of human LKB1 depends on membrane binding
As association with the membrane is essential for LKB1 to accomplish its function during development of Drosophila, we further investigated whether this mechanism is conserved throughout evolution and controls the activity of human LKB1 (hLKB1). Similar to its fly homologue, mutation of the polybasic region at the C terminus of hLKB1 together with mutation of the farnesylation motif (Fig. 4a) resulted in an impaired membrane localization in HeLa cells (Fig. 4b,c).
(a) Alignment of DmLKB1 and hLKB1 reveals several positively charged aa in the C terminus of hLKB1. Mutated residues are marked with bold lines. (b,c) GFP-hLKB1 localizes predominately to the nucleus and plasma membrane of HeLa cells cotransfected with STRADα (b), whereas GFP-hLKB1ΔLB C430A is not recruited to the cortex (c). (d,e) Immunoblottings of lysates from transfected HeLa cells, which lack substantial LKB1 expression, demonstrate an increase of activated AMPK (phospho-T172 AMPK), activated MARK (phospho-MARK) and decreased mTOR activation (measured by phosphorylation of S6K) upon GFP-hLKB1+STRADα transfection, which is not observed in GFP-hLKB1ΔLB C430A+STRADα transfected cells (d). Co-transfection of PLD2 but not of a catalytically reduced variant (PLDY511F) together with hLKB1 results in a further increase of AMPK activation (e). The intensity of pAMPK bands was quantified (normalized against total AMPK) (e, lower panel, control was set as 100%). (f) Cell viability of HeLa cells under energetic stress (induced by incubation with AICAR for 12 h) was estimated using the MTT assay (as described in the methods section). Scale bars are 10 μm. Experiments were performed in triplicates. Error bars represent s.d. and statistical significance was determined using ANOVA: P<0.01, **P<0.05, not significant (NS).
Activation of AMPK is one of the most important functions of LKB1 during tumour progression, which is further enhanced under energetic stress. As activated AMPK directly and indirectly inhibits mTOR8,9,10,11,12,13,14,15, the LKB1-AMPK pathway controls mTOR activity, e.g., upon aberrant activation of Akt, which occurs in various types of tumours with loss of function of PTEN or PI3-Kinase gain of function mutations37. Loss of membrane binding capacity of hLKB1 strongly decreases its ability to activate AMPK in HeLa cells (Fig. 4d), which lack substantial endogenous LKB1 expression, and consequently leads to apoptosis in metabolically stressed cells (Fig. 4f). Apart from AMPK activation, hLKB1ΔLB C430A fails to activate two other kinases of the AMPK-family, MARK (Fig. 4d) and SadA (Supplementary Fig. 5a). Furthermore, hLKB1ΔLB C430A is no longer able to inhibit mTOR (as estimated by phosphorylation of p70-S6-Kinase 1, pS6K, Fig. 4d). Vice versa, addition of PA-enriched liposomes to a recombinant hLKB1/hSTRADα/hMo25 complex enhanced autophosphorylation of hLKB1 and AMPK phosphorylation in vitro, whereas Phosphatidylcholine (PC) alone or PtdIns(4,5)P2 and PtdIns(3,4,5)P3-enriched liposomes had only a small effect on the activity of hLKB1 (Supplementary Fig. 3c,d).
Overexpression of PLD2 enhances LKB1 activity
PA is generated (among other pathways) from PC by two isoforms of PLD, PLD1 and 2 (ref. 38). Expression of PLD2 but not of a variant of PLD2 with a reduced catalytic activity (PLD2 Y511F (ref. 39)) together with hLKB1 results in a further increase of AMPK and MARK activation (Fig. 4e), suggesting that PLD2-induced production of PA enhances LKB1 activity in vivo. Conversely, inhibition of PLD reduces LKB1’s capacity to activate AMPK (Supplementary Fig. 5b). However, basal levels of activated AMPK are maintained, indicating that other enzymes (e.g., Diacylglycerol kinase) can compensate loss of PLD activity. This is in line with the observation that C. elegans, Drosophila and mouse mutants lacking PLD enzymes do not exhibit as dramatic phenotypes as LKB1ΔLB C564A (refs 40, 41, 42).
Membrane-binding-deficient LKB1 fails to induce multiple axons
Overexpression of LKB1 induces the extension of multiple axons43,44,45. Consistently, overexpression of wild-type GFP-hLKB1 together with STRADα results in the formation of multiple axons by the majority of transfected rat hippocampal neurons in culture (Fig. 5b,f). Remarkably, expression of farnesylation-deficient hLKB1C430A induces this phenotype as efficiently as wild-type LKB1 (Fig. 5c,f). By contrast, mutation of the C-terminal basic region (hLKB1ΔLB) almost abolished the effect on axon formation (Fig. 5d,f). Inactivation of the farnesylation signal in lipid-binding-deficient hLKB1 (hLKB1ΔLB C430A) did result in further reduction of LKB1 activity in this assay (Fig. 5d,f). These results confirm the importance of the C-terminal membrane binding domain for the function of LKB1 in mammalian cells.
(a–f) Hippocampal neurons from E18 rat embryos were transfected at 0 d.i.v. with vectors for GFP (control), GFP-LKB1 or GFP fusion proteins for the indicated LKB1 mutants and STRAD and analysed at 3 d.i.v by staining with the Tau-1 antibody (axonal marker). Representative images of transfected neurons are shown. The scale bar is 20 μm. (f) The development of neuronal polarity was analysed by counting the number of neurons without an axon (0, black), with a single axon (1, gray) or with multiple axons (>1, white). Most primary rat hippocampal neurons transfected with GFP alone (control) develop only a single axon (a, quantified in f). In contrast, neurons overexpressing GFP-hLKB1+STRADα or GFP-hLKB1C430A+STRADα (b,c,f) frequently establish two or more axons, whereas hLKB1 variants, which are deficient in membrane binding, fail to induce a multiple axon phenotype (d–f). Experiments were performed in triplicates. Error bars represent s.d. and statistical significance was determined using ANOVA: P<0.0001, ****P<0.01, **P>0.05, not significant (NS).
Expression of LKB1 is downregulated in malignant melanoma
As we have demonstrated that overexpression of PLD2 in cell culture results in increased activity of LKB1 (reflected by enhanced AMPK activation, Fig. 4e), we next assessed the pathophysiological relevance of our findings regarding tumour formation in vivo. Interestingly, expression of PLD2 is upregulated in several types of cancer, including melanoma, and high PLD2 expression correlates with poor survival rates of patients46,47. In cultured melanoma cells lacking detectable LKB1 expression (IGR37), membrane binding of LKB1 is essential for efficient AMPK (and MARK) activation, suppression of mTOR and cell survival under energetic stress (Supplementary Fig. 6).
Our data suggest that an aberrant increase in PLD expression in cancer cells will lead to increased PA levels and subsequent mTOR activation48,49,50 only if in addition the expression of LKB1 is reduced, because PA-mediated LKB1/AMPK activation normally counteracts mTOR activity. To test this hypothesis, we investigated whether increased expression of PLD, aberrant activation of Akt and decreased LKB1 expression correlate with enhanced mTOR activity in melanoma. Indeed, biopsies of melanoma primary tumours show a strong correlation between these four parameters. In 81% of all analysed tumours PLD2 expression as well as phospho-Akt and mTOR activity were elevated while the expression of LKB1 was decreased (Fig. 6a–e). Only 12% of the samples showed mTOR activation in the presence of stimulating phospho-Akt and PLD2-overexpression although LKB1 staining appeared normal, which might be explained by inactivating mutations of LKB1, which do not affect protein expression or stability. In contrast, melanocytes in biopsies of healthy skin exhibited a strong LKB1 expression, as well as low level of PLD2, phospho-Akt and mTOR activation (Fig. 6a–d, arrows). Melanocytic nevi, a benign proliferation of melanocytes, which can give birth to malignant melanoma, show in many cases elevated PLD2 expression as well as activation of Akt, whereas LKB1 expression is still high in most cases, suppressing aberrant mTOR activation, which occurred only in 25% of the analysed specimen (Fig. 6a’–d’). Thus in melanoma, loss of LKB1 in PLD2 overexpressing tumours may further contribute to mTOR activation, whereas in benign neoplasia higher LKB1 level counterbalance aberrant PLD2 activation.
Immunostainings of paraffin-embedded tissue sections of normal skin (a–d), neavi (a′–d′) and primary malignant melanoma tumours (a′′–d′′). (a) hLKB1 is highly expressed in melanocytes in the stratum basale of the epidermis in situ (a, arrows), and still detectable at substantial levels (a′) whereas it is almost undetectable in the majority of malignant melanoma (a′′). (b) PLD2 is not detectable in melanocytes of healthy skin sections (b, arrows) but becomes overexpressed in naevi (b′) and primary tumours (b′′). (c) Akt is activated (indicated by staining of phospho Akt, pAkt) in naevi (c′) and malignant melanoma (c′′), whereas it is not detectable in melanocytes of healthy skin samples (c). (d) Melanocytes in healthy skin biopsies (d) show only faint nuclear staining for phospho S6K (pS6K), which was used as to evaluate mTOR activation. Naevi (d′) exhibit only very few pS6K positive cells, whereas malignant melanoma show strong cytoplasmic and nuclear staining of pS6K (d′′). (e) Quantification of melanoma biopsies scored for the indicated correlations. ‘Others’ are tumours, which do not exhibit increased phospho-Akt or PLD2 staining.
Discussion
In this study, we have elucidated a conserved mechanism regulating LKB1 activity and function in vivo and during tumour suppression (Fig. 7). We have further established the first link between PLD-mediated production of PA and activation of LKB1. Our data suggest that membrane targeting of LKB1 by direct binding to PA is essential for the function of LKB1 during Drosophila development and for activation of AMPK and suppression of mTOR activity in cultured mammalian cells. Furthermore, downregulation of LKB1 in malignant melanoma specimen, which exhibit activated Akt and overexpression of PLD2, correlates with enhanced mTOR activity, indicating that the mechanism described in this study contributes to pathogenesis of malignant melanoma.
Under physiological conditions in non-tumourous cells, PA is produced by PLD (and other enzymes), resulting in an activation of mTOR and LKB1. Due to the inhibition of mTOR by LKB1-activated AMPK, the final result of increased PA level is a balanced cell proliferation and -growth. In contrast, in malignant melanoma, increased PI3K/Akt-signalling and enhanced production of PA by overexpression of PLD results in aberrant mTOR activation which is not counterbalanced by LKB1/AMPK and thus leads to increased cell proliferation and -growth and impaired tumour suppression.
In contrast to previous studies investigating the role of LKB1 farnesylation in the oocyte of Drosophila 22, we found farnesylation of LKB1 to be dispensable for the correct protein localization in the embryonic epidermis and NBs and for the function of LKB1 in Drosophila development. This observation is in line with previous findings from cultured mammalian cells and mice. In malignant melanoma cells, farnesylation-deficient LKB1 suppresses colony formation at similar degree as the wild-type kinase20. Furthermore, studies from knock-in mice show that mice homozygous for farnesylation deficient LKB1 are viable and do not exhibit any obvious phenotypes21. However, in these mice AMPK is less phosphorylated, whereas in cell culture experiments, no difference was found between wild-type and farnesylation deficient LKB1 (ref. 51). Notably, several C-terminally truncated hLKB1 variants (due to nucleotide deletions or point mutations in STK11) with an intact kinase domain have been reported52 and OMIM to be associated with Peutz-Jeghers syndrome, which might be due to the function of the C-terminal region in PA-mediated activation of LKB1.
Apart from the association of LKB1 with STRADα/Mo25, membrane binding and PA-mediated activation of LKB1 is a newly described upstream mechanism, which controls LKB1 kinase activity and function in vitro and in vivo. The regulation of LKB1 function by membrane recruitment raises the question whether this depends primarily on membrane localization or whether lipid binding itself stimulates LKB1 activity. Several (phospho)-lipids have been demonstrated to activate or increase the activity of various serine/threonine kinases, including Phosphoinositide-dependent kinase 1 (PDK1, activated by PtdIns(3,4,5)P3) or PKCζ, Raf1, and mTOR (all activated by PA49,53,54,55). Fusion of GFP-LKB1ΔLB C564A to lipid-binding domains (PH(PLCδ) and PH(Akt1)) resulted in a re-activation of GFP-LKB1ΔLB C564A (Fig. 1o; Supplementary Fig. 4). Furthermore, the activity of LKB1ΔLB C564A is strongly reduced in in vitro kinase assays (Fig. 3f,g). These findings could substantiate a hypothesis, in which the membrane functions as a scaffold for bringing together LKB1 and its cofactors (STRADα and Mo25) or its substrates. However, membrane binding deficient LKB1 robustly associates with its cofactors and one of its substrates (AMPK, Supplementary Fig. 3b). Moreover, addition of PA (but not of PtdIns(4,5)P2 or PtdIns(3,4,5)P3) is capable to substantially enhance the kinase activity of LKB1 in vitro (Fig. 3g; Supplementary Fig. 3c,d). Thus it is more likely, that in addition to membrane recruitment, PA functions to induce a conformational change of LKB1 thus enhancing its kinase activity. However we cannot exclude that in our in vitro kinase assays using immunoprecipiptated proteins from transfected cells wild-type LKB1 might have co-immunoprecipitated with PA-enriched micelles, which enhanced its kinase activity in this experiment.
Interestingly, PLD-produced PA activates both, LKB1 (this study) and mTOR48,49,50,55. Up to now, overexpression of PLD and increased levels of PA have been only assigned to pro-oncogenic pathways, in particular mTOR and Raf signalling54,55,56,57,58. Our data thus demonstrate the first anti-oncogenic mechanism of PLD function, which might counterbalance its pro-oncogenic effects as long as the LKB1-AMPK signalling axis is intact (Fig. 7).
Notably, Mukhopadhyay et al. showed recently, that in a mesenchymal-like breast cancer cell line (MDA-MB-231), which expresses robust levels of endogenous LKB1 (in contrast to the majority of breast cancer metastases), activated PLD induces a reduction in phospho-AMPK59. Thus, PLD-mediated activation of LKB1 might be either cell type specific or depends on the basal activation of both signalling cascades, the LKB1-AMPK axis and the PI3K-AKT pathway.
The LKB1-AMPK signalling pathway is well described to play a crucial role for cell polarity, energy homeostasis and cell proliferation and is frequently disturbed in various types of cancer16,17. As mentioned above, one particular function of AMPK is to counterbalance the pro-proliferative activity of mTOR8,9,10,11,12,13,14,15. Defects in the LKB1-AMPK signalling pathway (resulting in impaired inhibition of mTOR) have been already demonstrated to contribute to BRAF- and KRAS-induced melanoma formation in mouse models60,61,62,63. Remarkably, somatic mutations in human STK11 (encoding LKB1) are rather rare in malignant melanoma64,65. However, our data demonstrate that downregulation of LKB1 protein expression can be observed in a majority of malignant melanoma samples and therefore most likely contributes to the pathogenesis of this type of skin cancer.
Our study provides strong evidences for an important and conserved regulatory mechanism, which facilitates membrane recruitment of LKB1 by PA and is essential for the kinase activity and function of LKB1 during development and tumour suppression. The dissection of upstream regulatory mechanisms of LKB1 contributes to a better understanding of the physiological regulation of LKB1 and their misregulation in tumours, which might be useful for the development of therapeutic approaches in the future.
Methods
Fly stocks and genetics
UASt::GFP-LKB1 and lkb1::GFP-LKB1 transgenes were generated using phiC31-mediated germ line transformation on attp40. For rescue experiments of different LKB1 variants, we used the lkb1 x5 null allele. Lethality tests were performed in three independent experiments with n=100 in each experiment. For rescue experiments using the UAS/GAL4 system, we used UAS::GFP-LKB1 and actin5C::GAL4 instead of lkb1::GFP-LKB1.
DNA and constructs
Cloning of the cDNA of wild-type LKB1 into pENTR was performed using standard PCR on a full length EST clone (Drosophila Genomics Resources Center, DGRC) as template using the following primers: LKB1-F: 5′- CACCATGCAATGTTCTAGCTCTCGG-3′, LKB1-R: 5′-CTACGAAGTTCGGCAGTGG-3′. Similar, truncated fragments of LKB1 were cloned with the following oligonucleotides: LKB1512-F: 5′-CACCATGCACACCTACGAACCGCC-3′, LKB1536-F: 5′-CACCATGGCGCCCGTCAAGAAG-3′, LKB1552 -F: 5′-CACCATGCTGACGTCCTGCATCTCCG-3′. For expression of LKB1 from its endogenous promoter we inserted a genomic fragment (from 2.8 kbp upstream of the translation start to 1 kbp downstream of the stop codon) into pENTR using the following primers: LKB1gen-F: 5′- CACC CACTAGCGTAATTTGACGG-3′, LKB1gen-R: 5′- CTC GAG CAGCAGTACGGTCATCTC-3′. An XbaI-cutting site was introduced replacing the start codon using mutagenesis PCR and the following primer: LKB1gen-XbaI-F: 5′-GGCTCCGCGGAGGTTTTCTAGACAATGTTCTAGCTCTC-3′. Subsequently, GFP was inserted into the XbaI site by PCR and standard ligation. Mutagenesis PCR was used to generate defined point mutations with full length or genomic LKB1 cDNA in pENTR as template. The following oligonucleotides were used for mutagenesis (mutation underlined): LKB1ΔLB: Combination of 1. LKB1K546A R547A R548A K550A K551A-F: 5′-TCGGCACTGGCGGCGGCCGCCGCGGCGCTGACGTCCTGC-3′ and 2.LKB1K539A K540A K541A-F: 5′-GAGGAGGCGCCCGTCGCCGCGGCGGGATCGGCACTG-3′, LKB1C564A-F: 5′-GTGCGCAAGCTTAGCCACGCCCGAACTTCGTAG, LKB1D317A-F: 5′- CAAACGCTGAAGATTTCCGCCTTCGGTGTGGCG.
To express LKB1ΔLBC564A PH(PLD) and LKB1ΔLBC564A PH(Akt), the PH domain of PLCδ and Akt was amplified by PCR and ligated via an endogenous Bpu1101-I site into lkb1::LKB1 pENTR using the following primers: PH(PLCδ)-F: 5′- GCTGAGCCACGCCCGAACT TCGgatgaggatctacaggcgct-3′, PH(PLCδ)-R: 5′- GCTGAGCTAGATCTTGTGCAGCCCCAG-3′, PH(Akt)-F: 5′-GCTGAGCCACGCCCGAACTTCGGTCGTAAAGGAGGGGTGG-3′ and PH(Akt)-R: 5′- GCTGAGCTTATATGAGCCGGCTGGATAC-3′.
For expression of hLKB1, the open reading frame of hLKB1 was cloned into pENTR using the following nucleotides: hLKB1-F: 5′- CACC ATGGAGGTGGTGGACCC-3′ and hLKB1-R: 5′- TCACTGCTGCTTGCAGG-3′. For mutation of the farnesylation, lipid binding motif and construction of the kinase dead version, the following nucleotides were used in mutagenesis PCRs: hLKB1C430A-F: 5′- CGCCGGCTGTCGGCCGCTAAGCAGCAGTGAAAGGGT-3′, hLKB1R415AK416A-F: 5′- GCCCCCAACCCTGCCGCCGCGGCCTGCTCCGCCAGC-3′ and hLKB1D194A: 5′- ACCCTCAAAATCTCCGCCCTTGGCGTGGCCGAGGCA-3′.
Constructs were recloned into GFP-tagged destination vectors containing a One-Strep-Tag fused to the N terminus of GFP (USGW, modified TGW, Murphy lab, DGRC, expression in S2R cells) or into a modified EGFP-C1 vector (CGW, expression in mammalian cells) containing a gateway cassette using the gateway technology (Life technology).
Antibodies
Antisera directed against full length LKB1 were raised by injection of a fusion protein of LKB1 and MBP into two guinea pigs (Amsbio, Abingdon, UK).
Cell culture and cell viability assay
HeLa, IMR90, IGR37 and MDCK cells were obtained from ATCC. All cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, 2 mM Glutamine at 37 °C in a 5% CO2 atmosphere and negatively tested for mycoplasma contamination by PCR. Cells were transfected using FUGENE (Promega) according to the manufacturer’s instructions. For evaluation of cell viability upon energetic stress, 50 × 103 cells/well were transiently transfected in a 24well plate with GFP-hLKB1+STRADα constructs and treated for 12 h with 2.5 mM AICAR (Santa Cruz Inc.). GFP+STRADα were used as negative control. Cell viability was assessed in triplicates using MTT assay according to the manufacturer’s instructions (SIGMA).
Western blotting and coimmunoprecipitation
Western blotting was done as previously described66. For mammalian cell culture experiments, HeLa or IGR37 cells were transiently transfected with the indicated constructs. 48 hours after transfection, cells were incubated with 2 mM AICAR for 1 h and harvested in lysis buffer (1% Triton X-100, 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 50 mM TRIS-HCl pH 7.5) supplemented with protease- and phosphatase inhibitors. For embryonic lysates, lkb1::GFP-LKB1 expressing embryos were collected from overnight plates. Coimmunoprecipitation using GFP-Trap (ChromoTek) of GFP-LKB1 with HA-tagged DmSTRADα (Stlk) and myc-tagged DmMo25 was done in embryonic lysates, which ubiquitously expressed the proteins using arm::GAL4. Primary antibodies used for western blotting were as follows: rabbit anti-Actin (1:1,000, sc-47778, Santa Cruz), mouse anti-S6K (1:500, sc-8418, Santa Cruz ), rabbit phosphoT389-S6K (1:500, sc-11759, Santa Cruz), rabbit anti-phospho-T172-AMPK (1:200, sc-33524, Santa Cruz), rabbit anti-phospho-MARK1/2/3 (1:500, PA5-17495, Thermo Scientific), mouse anti-myc (1:100, 9E10, DSHB), rabbit anti-AMPK (1:400, sc-25792, Santa Cruz), guinea pig anti LKB1 (1:500, this study), mouse anti-GFP (1:500, sc-9996, Santa Cruz), mouse anti-HA (1:500, #11583816001, Roche), rabbit anti-GST (1:5,000, #G7781, SIGMA). For statistical analysis, three independent experiments were scored. Intensity of the bands was quantified by ImageJ.
Immunohistochemistry
Drosophila embryos were fixed in 4% formaldehyde, phosphate buffer pH 7.4 as described before67. Primary antibodies used for indirect immunofluorescence were as follows: guinea pig anti LKB1 (1:250, this study), rabbit anti Baz (1:2,000, kindly provided by A. Wodarz), mouse anti α-spectrin (3A9, 1:50, DSHB), mouse anti Dlg (4F10, 1:50, DSHB), rat anti DE-Cad (DCAD2, 1:25, DSHB), rabbit anti GFP (1:400, sc-8334, Santa Cruz Inc), guinea-pig anti Miranda (1:1,000, kindly provided by A. Wodarz). HeLa cells were fixed with 4% PFA in PBS and stained with the rabbit anti-phospho-Sad antibody (1:250) and a mouse anti-LKB1 antibody (1:200, sc-32245, Santa Cruz) in 10% goat serum.
Secondary antibodies conjugated with Alexa 488, Alexa 568 and Alexa 647 (Life Technology) were used at 1:400. Images were taken on a Zeiss LSM 710 Meta confocal microscope and processed using Adobe Photoshop.
For immunohistochemical staining of healthy skin, nevi and melanoma, a tissue micro array (TMA) of paraffin-embedded healthy skin, nevi and melanoma primary tumours was analysed. Paraffin sections were deparaffinized for 30 min at 72 °C, washed two times for 7 min in Xylol and subsequently re-watered in a descending sequence of ethanol/water mixture. Prior to staining sections were subjected for 5 min to heat-induced epitope-retrieval (HIER) using 1 mM Tris-EDTA-buffer (pH 8.5) at 120 °C. Sections were blocked in peroxidase-blocking solution (Dako, #S2023) for 5 min at room-temperature and washed 5 min with wash buffer (Dako, #S3006) prior to incubation with primary antibody diluted in antibody diluent (Dako, #S2022) for 30 min. Primary antibodies were as follows: rabbit anti-LKB1 (D60C5F10, 1:200, Cell Signaling), rabbit anti-phospho-Akt (1:20, Cell Signaling #4060), rabbit anti-PLD2 (1:1,000, Cell Signaling #13891) and rabbit anti-S6K-phospho-T389 S6K (1:50, Cell Signaling #9206). Subsequently, sections were washed with wash buffer and incubated for 30 min with HRP-coupled secondary antibody (Dako EnVision, # K5007). Stainings were developed after washing using DAB/chromogen solution (Dako, #K5007). To visualize cellular structures, stained sections were counterstained with hematoxylin (Merk, #10517505000) for 1 min and dehydrated in ethanol/xylol before embedding. The staining intensity was determined blinded for all tissue samples as followed: negative-0, weak-+, moderate-++ and strong-+++. LKB1 exhibited a strong expression in melanocytes in situ, so weak or negative intensity was scored as downregulation of the protein, whereas moderate staining was classified as slightly downregulated. Activated Akt was negative in healthy skin biopsies, so weak, moderate and strong staining was classified as upregulated. PLD2 expression was negative or weak in melanocytes in situ, thus moderate and strong staining was scored as upregulated. Phospho-S6K was strongly expressed in the nucleus but not in the cytoplasm of melanocytes but cytoplasmic staining occurred only in malignant tumours. Consequently, weak to strong cytoplasmic staining of phospho-S6K was classified as upregulated.
Lipid binding assays
Fusion proteins of the C terminus of LKB1 (aa 353–567) with MBP were expressed in E.coli and affinity purified. Lipid strips (Echelon) were incubated over night with purified MBP-LKB1 fusion proteins at 0.5 μg ml−1 in TBST containing 3% BSA, washed and probed with antibodies against MBP (1:1,000, Santa Cruz sc-73416) as described above.
For membrane floatation and in vitro kinase assays, lipids were obtained from Avanti Polar Lipids (Egg-PA, #840101, Egg-PC #840051, Brain PtdIns(4,5)P2 #840046) and Echelon (PtdIns(3,4,5)P3 #P-3916). Liposomes (10 mM total lipid concentration, either PC alone or PC:PA/PtdIns(3,4,5)P3/PtdIns(4,5)P2 in a 9:1 molar ratio) were prepared in LB buffer (30 mM Tris, 4 mM EGTA, pH 8.0) by extrusion through a 0.1 μm polycarbonate membrane using a Mini-Extruder (Avanti Polar Lipids). The membrane floatation was performed as follows68: liposomes (100 μl of 10 mM total lipid concentration) were incubated on ice for 30 min with 1 μg recombinant protein. LB buffer (30 mM Tris, 4 mM EGTA, 2 M sucrose (pH 8.0)) was added to the incubation reaction to bring the final sucrose concentration to 1.6 M, and this mixture was overlaid with cushions containing 1.4 M, 0.4 M, and 0.25 M sucrose in the same buffer in a TLA-55 tube. After centrifugation at 186,000g (4 °C) for 45 min in a TLA-55 rotor (Beckman), the 0.25/0.4 M interphase (top fraction, T) and the loading fraction (bottom fraction, B) were collected and analysed by SDS-PAGE and western blot. For statistical analysis, three independent experiments were scored. Intensity of the bands was quantified by ImageJ.
In vitro kinase assay
OneStrep-GFP-LKB1 plus OneStrep-GFP-Stlk were precipitated from transfected S2R cells (2 mg total protein lysates) using Streptactin beads (IBA, Goettingen, Germany). The beads were washed five times in harsh washing buffer (50 mM TRIS pH 7.5, 500 mM NaCl) and one time in LKB1 kinase buffer (50 mM TRIS pH 7.5, 10 mM MgCl2, 10 mM MnCl2, 1 mM DTT, 100 μM ATP, phosphatase- and protease inhibitors). Immunoprecipitated proteins were then incubated with 2 μg of recombinant GST-DmAMPKα108-280 (=aa 108–280, containing the T-loop with the LKB1-phosphorylation site) and 0.3 μCi[γ-32ATP] in kinase buffer for 1 h at 30 °C. The reaction was terminated by addition of SDS sample buffer and samples were subjected to SDS-PAGE. Phosphorylation was detected by exposure to X-ray films and quantified with Aida 2D Densitometry software.
For assays with human recombinant kinase complex (Supplementary Fig. 3b,c), 1 μg of a complex of recombinant hLKB1, STRADα and Mo25 (SIGMA) was used as described above replacing immunoprecipitated LKB1 protein. In this experiment, GFP-AMPKα1 from transfected HeLa cells (2 mg total protein lysate) was used as substrate as a recombinant fragment would have interfered with the autophosphorylation bands. After isolation of precipitated OneStrep-GFP-AMPK, recombinant hLKB1/STRADα/Mo25 was added and incubated as described above. Subsequently, OneStrep-GFP-AMPK was purified from the reaction mixture using Streptactin beads.
For kinase assays with addition of lipids, Liposomes were prepared as described above and added at 10 mM final concentration.
For statistical analysis, three independent experiments were scored. Intensity of the bands was quantified by ImageJ.
Transfection and analysis of neurons
Hippocampal neurons were isolated from the brains of E18 rat embryos and cultured as described previously69. Dissociated hippocampal neurons were plated at 70,000 cells per well in a 24 well plate and transfected 3 h after plating by calcium phosphate co-precipitation. Neurons were fixed at 3 DIV and permeabilized with 0.1% Triton X-100, 0.1% sodium citrate in PBS for 3 min on ice. Neurons were stained with the Tau-1 antibody as axonal marker (Chemicon, MAB3420; 1:300) and Alexa-Fluor-conjugated secondary antibodies (Molecular Probes; 1:300). The stage of neuronal differentiation and axon formation was determined according to published criteria70. For each LKB1 variant, 3 different experiments with 100 analysed neurons in each were scored.
Statistics
All experiments were performed in triplicates. Error bars represent s.d. and statistical significance was determined using ANOVA: P<0.0001, ****P<0.001, ***P<0.01, **P<0.05, *P>0.05, not significant (NS).
Data availability
The authors declare that all data are available within the Article and Supplementary Files, or available from the authors upon request.
Additional information
How to cite this article: Dogliotti, G. et al. Membrane-binding and activation of LKB1 by phosphatidic acid is essential for development and tumour suppression. Nat. Commun. 8, 15747 doi: 10.1038/ncomms15747 (2017).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
08 March 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41467-022-28923-3
References
Lizcano, J. M. et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 23, 833–843 (2004).
Vaahtomeri, K. & Makela, T. P. Molecular mechanisms of tumor suppression by LKB1. FEBS Lett. 585, 944–951 (2011).
Shaw, R. J. et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl Acad. Sci. USA 101, 3329–3335 (2004).
Lee, J. H. et al. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature 447, 1017–1020 (2007).
van der Velden, Y. U. et al. The serine-threonine kinase LKB1 is essential for survival under energetic stress in zebrafish. Proc. Natl Acad. Sci. USA 108, 4358–4363 (2011).
Hawley, S. A. et al. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2, 28 (2003).
Woods, A. et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 13, 2004–2008 (2003).
Inoki, K., Zhu, T. & Guan, K. L. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115, 577–590 (2003).
Shaw, R. J. et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell 6, 91–99 (2004).
Gwinn, D. M. et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 30, 214–226 (2008).
Arsham, A. M., Howell, J. J. & Simon, M. C. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J. Biol. Chem. 278, 29655–29660 (2003).
Kimura, N. et al. A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling pathway. Genes Cells 8, 65–79 (2003).
Shackelford, D. B. et al. mTOR and HIF-1alpha-mediated tumor metabolism in an LKB1 mouse model of Peutz-Jeghers syndrome. Proc. Natl Acad. Sci. USA 106, 11137–11142 (2009).
Corradetti, M. N., Inoki, K., Bardeesy, N., DePinho, R. A. & Guan, K. L. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev. 18, 1533–1538 (2004).
Shaw, R. J. et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310, 1642–1646 (2005).
Hardie, D. G. & Alessi, D. R. LKB1 and AMPK and the cancer-metabolism link—ten years after. BMC Biol. 11, 36 (2013).
Nakano, A. & Takashima, S. LKB1 and AMP-activated protein kinase: regulators of cell polarity. Genes Cells 17, 737–747 (2012).
Boudeau, J. et al. MO25alpha/beta interact with STRADalpha/beta enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J. 22, 5102–5114 (2003).
Dorfman, J. & Macara, I. G. STRADalpha regulates LKB1 localization by blocking access to importin-alpha, and by association with Crm1 and exportin-7. Mol. Biol. Cell. 19, 1614–1626 (2008).
Sapkota, G. P. et al. Phosphorylation of the protein kinase mutated in Peutz-Jeghers cancer syndrome, LKB1/STK11, at Ser431 by p90(RSK) and cAMP-dependent protein kinase, but not its farnesylation at Cys(433), is essential for LKB1 to suppress cell vrowth. J. Biol. Chem. 276, 19469–19482 (2001).
Houde, V. P. et al. Investigation of LKB1 Ser431 phosphorylation and Cys433 farnesylation using mouse knockin analysis reveals an unexpected role of prenylation in regulating AMPK activity. Biochem. J. 458, 41–56 (2014).
Martin, S. G. & St Johnston, D. A role for Drosophila LKB1 in anterior-posterior axis formation and epithelial polarity. Nature 421, 379–384 (2003).
Song, P. et al. Protein kinase Czeta-dependent LKB1 serine 428 phosphorylation increases LKB1 nucleus export and apoptosis in endothelial cells. J. Biol. Chem. 283, 12446–12455 (2008).
Tiainen, M., Ylikorkala, A. & Makela, T. P. Growth suppression by Lkb1 is mediated by a G(1) cell cycle arrest. Proc. Natl Acad. Sci. USA 96, 9248–9251 (1999).
Smith, D. P., Spicer, J., Smith, A., Swift, S. & Ashworth, A. The mouse Peutz-Jeghers syndrome gene Lkb1 encodes a nuclear protein kinase. Hum. Mol. Genet. 8, 1479–1485 (1999).
Baas, A. F. et al. Activation of the tumour suppressor kinase LKB1 by the STE20-like pseudokinase STRAD. EMBO J. 22, 3062–3072 (2003).
Sebbagh, M., Santoni, M. J., Hall, B., Borg, J. P. & Schwartz, M. A. Regulation of LKB1/STRAD localization and function by E-cadherin. Curr. Biol. 19, 37–42 (2009).
Bonaccorsi, S. et al. The Drosophila Lkb1 kinase is required for spindle formation and asymmetric neuroblast division. Development 134, 2183–2193 (2007).
Watts, J. L., Morton, D. G., Bestman, J. & Kemphues, K. J. The C. elegans par-4 gene encodes a putative serine-threonine kinase required for establishing embryonic asymmetry. Development 127, 1467–1475 (2000).
Collins, S. P., Reoma, J. L., Gamm, D. M. & Uhler, M. D. LKB1, a novel serine/threonine protein kinase and potential tumour suppressor, is phosphorylated by cAMP-dependent protein kinase (PKA) and prenylated in vivo. Biochem. J. 345, (Pt 3): 673–680 (2000).
McTaggart, S. J. Isoprenylated proteins. Cell. Mol. Life Sci. 63, 255–267 (2006).
Stace, C. L. & Ktistakis, N. T. Phosphatidic acid- and phosphatidylserine-binding proteins. Biochim. Biophys. Acta 1761, 913–926 (2006).
Varnai, P. & Balla, T. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J. Cell Biol. 143, 501–510 (1998).
Pawelczyk, T. & Matecki, A. Phospholipase C-delta3 binds with high specificity to phosphatidylinositol 4,5-bisphosphate and phosphatidic acid in bilayer membranes. Eur. J. Biochem. 262, 291–298 (1999).
Klippel, A., Kavanaugh, W. M., Pot, D. & Williams, L. T. A specific product of phosphatidylinositol 3-kinase directly activates the protein kinase Akt through its pleckstrin homology domain. Mol. Cell. Biol. 17, 338–344 (1997).
Bruntz, R. C., Taylor, H. E., Lindsley, C. W. & Brown, H. A. Phospholipase D2 mediates survival signaling through direct regulation of Akt in glioblastoma cells. J. Biol. Chem. 289, 600–616 (2014).
Menon, S. & Manning, B. D. Common corruption of the mTOR signaling network in human tumors. Oncogene 27, S43–S51 (2008).
Gomez-Cambronero, J. Phospholipase D in cell signaling: from a myriad of cell functions to cancer growth and metastasis. J. Biol. Chem. 289, 22557–22566 (2014).
Henkels, K. M., Short, S., Peng, H. J., Di Fulvio, M. & Gomez-Cambronero, J. PLD2 has both enzymatic and cell proliferation-inducing capabilities, that are differentially regulated by phosphorylation and dephosphorylation. Biochem. Biophys. Res. Commun. 389, 224–228 (2009).
LaLonde, M. M. et al. Regulation of phototransduction responsiveness and retinal degeneration by a phospholipase D-generated signaling lipid. J. Cell Biol. 169, 471–479 (2005).
Sato, T., Hongu, T., Sakamoto, M., Funakoshi, Y. & Kanaho, Y. Molecular mechanisms of N-formyl-methionyl-leucyl-phenylalanine-induced superoxide generation and degranulation in mouse neutrophils: phospholipase D is dispensable. Mol. Cell. Biol. 33, 136–145 (2013).
Raghu, P., Manifava, M., Coadwell, J. & Ktistakis, N. T. Emerging findings from studies of phospholipase D in model organisms (and a short update on phosphatidic acid effectors). Biochim. Biophys. Acta 1791, 889–897 (2009).
Barnes, A. P. et al. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell 129, 549–563 (2007).
Shelly, M., Cancedda, L., Heilshorn, S., Sumbre, G. & Poo, M. M. LKB1/STRAD promotes axon initiation during neuronal polarization. Cell 129, 565–577 (2007).
Veleva-Rotse, B. O. et al. STRAD pseudokinases regulate axogenesis and LKB1 stability. Neural Dev. 9, 5 (2014).
Saito, M. et al. Expression of phospholipase D2 in human colorectal carcinoma. Oncol. Rep. 18, 1329–1334 (2007).
Henkels, K. M., Boivin, G. P., Dudley, E. S., Berberich, S. J. & Gomez-Cambronero, J. Phospholipase D (PLD) drives cell invasion, tumor growth and metastasis in a human breast cancer xenograph model. Oncogene 32, 5551–5562 (2013).
Yoon, M. S. et al. Rapid mitogenic regulation of the mTORC1 inhibitor, DEPTOR, by phosphatidic acid. Mol. Cell. 58, 549–556 (2015).
Fang, Y., Vilella-Bach, M., Bachmann, R., Flanigan, A. & Chen, J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science 294, 1942–1945 (2001).
Foster, D. A., Salloum, D., Menon, D. & Frias, M. A. Phospholipase D and the maintenance of phosphatidic acid levels for regulation of mammalian target of rapamycin (mTOR). J. Biol. Chem. 289, 22583–22588 (2014).
Denison, F. C., Hiscock, N. J., Carling, D. & Woods, A. Characterization of an alternative splice variant of LKB1. J. Biol. Chem. 284, 67–76 (2009).
Boudeau, J., Sapkota, G. & Alessi, D. R. LKB1, a protein kinase regulating cell proliferation and polarity. FEBS Lett. 546, 159–165 (2003).
Limatola, C., Schaap, D., Moolenaar, W. H. & van Blitterswijk, W. J. Phosphatidic acid activation of protein kinase C-zeta overexpressed in COS cells: comparison with other protein kinase C isotypes and other acidic lipids. Biochem. J. 304, (Pt 3): 1001–1008 (1994).
Ghosh, S., Strum, J. C., Sciorra, V. A., Daniel, L. & Bell, R. M. Raf-1 kinase possesses distinct binding domains for phosphatidylserine and phosphatidic acid. Phosphatidic acid regulates the translocation of Raf-1 in 12-O-tetradecanoylphorbol-13-acetate-stimulated Madin-Darby canine kidney cells. J. Biol. Chem. 271, 8472–8480 (1996).
Toschi, A. et al. Regulation of mTORC1 and mTORC2 complex assembly by phosphatidic acid: competition with rapamycin. Mol. Cell. Biol. 29, 1411–1420 (2009).
Zheng, Y. et al. Phospholipase D couples survival and migration signals in stress response of human cancer cells. J. Biol. Chem. 281, 15862–15868 (2006).
Shi, M., Zheng, Y., Garcia, A., Xu, L. & Foster, D. A. Phospholipase D provides a survival signal in human cancer cells with activated H-Ras or K-Ras. Cancer. Lett. 258, 268–275 (2007).
Rizzo, M. A. et al. Phospholipase D and its product, phosphatidic acid, mediate agonist-dependent raf-1 translocation to the plasma membrane and the activation of the mitogen-activated protein kinase pathway. J. Biol. Chem. 274, 1131–1139 (1999).
Mukhopadhyay, S. et al. Reciprocal regulation of AMP-activated protein kinase and phospholipase D. J. Biol. Chem. 290, 6986–6993 (2015).
Damsky, W. et al. mTORC1 activation blocks BrafV600E-induced growth arrest but is insufficient for melanoma formation. Cancer Cell 27, 41–56 (2015).
Liu, W. et al. LKB1/STK11 inactivation leads to expansion of a prometastatic tumor subpopulation in melanoma. Cancer Cell 21, 751–764 (2012).
Zheng, B. et al. Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation. Mol. Cell. 33, 237–247 (2009).
Esteve-Puig, R., Canals, F., Colome, N., Merlino, G. & Recio, J. A. Uncoupling of the LKB1-AMPKalpha energy sensor pathway by growth factors and oncogenic BRAF. PLoS ONE 4, e4771 (2009).
Guldberg, P. et al. Somatic mutation of the Peutz-Jeghers syndrome gene, LKB1/STK11, in malignant melanoma. Oncogene 18, 1777–1780 (1999).
Rowan, A. et al. Somatic mutations in the Peutz-Jeghers (LKB1/STKII) gene in sporadic malignant melanomas. J. Invest. Dermatol. 112, 509–511 (1999).
Cidlinsky, N. et al. Inactivation of the LKB1-AMPK signaling pathway does not contribute to salivary gland tumor development—a short report. Cell Oncol. 39, 389–396 (2016).
Sen, A., Nagy-Zsver-Vadas, Z. & Krahn, M. P. Drosophila PATJ supports adherens junction stability by modulating Myosin light chain activity. J. Cell Biol. 199, 685–698 (2012).
Krahn, M. P., Klopfenstein, D. R., Fischer, N. & Wodarz, A. Membrane targeting of Bazooka/PAR-3 is mediated by direct binding to phosphoinositide lipids. Curr. Biol. 20, 636–642 (2010).
Yang, R., Kong, E., Jin, J., Hergovich, A. & Puschel, A. W. Rassf5 and Ndr kinases regulate neuronal polarity through Par3 phosphorylation in a novel pathway. J. Cell. Sci. 127, 3463–3476 (2014).
Schwamborn, J. C. & Puschel, A. W. The sequential activity of the GTPases Rap1B and Cdc42 determines neuronal polarity. Nat. Neurosci. 7, 923–929 (2004).
Acknowledgements
We thank A. Wodarz, B. Schmidt, G. Längst, the Bloomington Drosophila stock center at the University of Indiana and the Developmental Studies Hybridoma Bank at the University of Iowa for providing reagents. This work was supported by grants of the DFG to M.P.K. (DFG3901/2-1, DFG3901/1-2, SFB699/A13) and to A.W.P. (DFG PU 102 12-1 and Cells-in-Motion Cluster of Excellence (EXC 1003—CiM)).
Author information
Authors and Affiliations
Contributions
G.D., L.K., C.T., P.D., O.P. and G.M. designed and performed the experiments, A.W.P. and M.P.K. designed and directed the project and wrote the manuscript. All authors discussed results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures and Supplementary Reference (PDF 910 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Dogliotti, G., Kullmann, L., Dhumale, P. et al. Membrane-binding and activation of LKB1 by phosphatidic acid is essential for development and tumour suppression. Nat Commun 8, 15747 (2017). https://doi.org/10.1038/ncomms15747
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms15747
This article is cited by
-
The exocyst complex and intracellular vesicles mediate soluble protein trafficking to the primary cilium
Communications Biology (2024)
-
Activity-based directed evolution of a membrane editor in mammalian cells
Nature Chemistry (2023)
-
PIM kinases inhibit AMPK activation and promote tumorigenicity by phosphorylating LKB1
Cell Communication and Signaling (2021)
-
The diversity and breadth of cancer cell fatty acid metabolism
Cancer & Metabolism (2021)
-
Adenosine A2A receptor signaling promotes FoxO associated autophagy in chondrocytes
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.