Abstract
Cotranslational chaperones assist in de novo folding of nascent polypeptides in all organisms. In yeast, the heterodimeric ribosome-associated complex (RAC) forms a unique chaperone triad with the Hsp70 homologue Ssb. We report the X-ray structure of full length Ssb in the ATP-bound open conformation at 2.6 Å resolution and identify a positively charged region in the α-helical lid domain (SBDα), which is present in all members of the Ssb-subfamily of Hsp70s. Mutational analysis demonstrates that this region is strictly required for ribosome binding. Crosslinking shows that Ssb binds close to the tunnel exit via contacts with both, ribosomal proteins and rRNA, and that specific contacts can be correlated with switching between the open (ATP-bound) and closed (ADP-bound) conformation. Taken together, our data reveal how Ssb dynamics on the ribosome allows for the efficient interaction with nascent chains upon RAC-mediated activation of ATP hydrolysis.
Similar content being viewed by others
Introduction
Already during translation at the ribosome, the emerging polypeptide chain is subject to enzymes for covalent modification, targeting factors for localization and chaperones for de novo protein folding1,2,3. In eukaryotes, the nascent polypeptide chain is modified by methionine amino-peptidases and N-acetyl transferases2,4,5. For targeting and insertion of membrane or secretory proteins into the endoplasmic reticulum membrane, the nascent chain is recognized and guided by the signal recognition particle (SRP) (refs 1, 6, 7, 8). To prevent aggregation and assist in folding of newly synthesized proteins, Hsp70 chaperones interact with nascent chains. Members of the Hsp70 family are highly conserved among all kingdoms of life and are known to bind short, largely hydrophobic sequences exposed by unfolded proteins9.
Hsp70 proteins share a number of structural and functional characteristics. They consist of a highly conserved N-terminal ATPase domain, also termed nucleotide binding domain (NBD), which is connected via a linker to the substrate binding domain (SBD). The SBD is divided into a β sandwich subdomain (SBDβ), an α-helical subdomain (SBDα or lid domain) and a less-conserved C-terminal domain of variable length9. The Hsp70–protein substrate interaction depends on ATP binding and on allosteric regulation between the NBD and the SBD. The ATP-bound state is characterized by a fast exchange rate of substrate (low affinity state), while in the ADP-bound state exchange is much slower (high affinity state)10. During the Hsp70 cycle, the chaperone switches between the ATP-bound state (open conformation) and the ADP-bound state (closed conformation) by major conformational rearrangements involving mainly the SBDα (refs 10, 11, 12, 13). Two types of cochaperones regulate the switch between the two states: Hsp40s stimulate ATP hydrolysis, and nucleotide exchange factors accelerate the exchange of ADP with ATP (refs 9, 14). Due to the high conservation, the allosteric mechanism recently established for DnaK likely applies to all canonical members of the Hsp70 family15.
Fungi from the large division of Ascomycota possess an evolutionary conserved subfamily of Hsp70s, termed the Ssb-type Hsp70s, which is named after two nearly identical homologues Ssb1 and Ssb2 (collectively Ssb) from Saccharomyces cerevisiae16. Ssb is distinguished from other Hsp70 homologues as it interacts directly with the ribosome by a mechanism that is currently unknown3,17. When bound to the ribosome, Ssb interacts with a large variety of nascent polypeptides and assists their cotranslational folding18. Because the ratio of ribosomes and Ssb in a yeast cell is near-balanced19 each ribosome could cooperate with one Ssb molecule throughout protein synthesis. However, only about 50% of Ssb is ribosome-associated at steady state, while the remainder is free in the cytosol3. This cytosolic pool of Ssb is involved in additional functions, not directly connected to cotranslational protein folding3,20,21.
Ribosome-bound Ssb cooperates with a specific cochaperone termed the ribosome-associated complex (RAC), which is composed of the Hsp70 protein Ssz1 and the Hsp40 protein Zuo1 (refs 22, 23, 24). Deletion of either ZUO1, SSZ1 or SSB1/SSB2 results in similar growth defects including slow growth, cold sensitivity and hypersensitivity towards paromomycin. The combined deletion of any of the above genes does not result in additive effects, suggesting that Ssb, Zuo1 and Ssz1 function together in the same pathway3,25,26. Of note the Ssb chaperone system is not induced at high temperature, but rather SSB1 and SSB2 are down-regulated together with ribosomal proteins on heat shock3,27. Rather than being heat shock proteins Ssb and RAC belong to the group of chaperones linked to protein synthesis3,28. The ribosome interaction of RAC has been characterized biochemically and structurally22,29,30,31. RAC binds close to the tunnel exit with contacts to Rpl22 and Rpl31 (refs 29, 30, 31), and several expansion segments of ribosomal RNA (rRNA), and stretches across the surface of the ribosome to the 40S ribosomal subunit31. Although the precise position of, for example the NBD of Ssz1 or the J-domain of Zuo1 are not resolved in the cryo-EM structures, the crosslink of Zuo1 to Rpl31 supports the idea that the J-domain is positioned close to the tunnel exit and possibly to Rpl31 (ref. 29). As the J-domain of Zuo1 activates the Ssb ATPase and switches it to the high affinity substrate binding state32, Ssb and the J-domain of Zuo1 should occupy neighbouring binding sites at the ribosome.
Here, we present a detailed structural and biochemical analysis of the Ssb–ribosome interaction. We determined the structure of full-length Ssb from the thermophilic Ascomycota Chaetomium thermophilum33,34 in the open ATP-bound conformation and characterized its ribosome binding by crosslinking and mutagenesis experiments. Ssb is positioned on the ribosome by dual interaction with ribosomal proteins and rRNA in close proximity of the tunnel exit. Ssb binding is modulated by RAC and our data allow to derive a model for the positioning and the dynamics of Ssb at the ribosome.
Results
Ssb interacts with ribosomal proteins at the tunnel exit
To better characterize the interaction of Ssb with ribosomes, we first investigated its positioning via crosslinking experiments. To this end, we employed a Δssb1Δssb2 strain expressing the point mutant Ssb1-A577K (termed Ssb1*), which displayed enhanced affinity for αSsb (Supplementary Fig. 1a). Ssb1* was ribosome associated and fully complemented growth of a Δssb1Δssb2 strain (Supplementary Fig. 1b,c). Crosslinking in cell extract generated several crosslink products detected by αSsb (Fig. 1a). After separation of the cytosol from ribosomes via a sucrose cushion, a prominent crosslink of ∼160 kDa was detected in the cytosolic fraction, while additional crosslinks of weaker intensity were observed in the ribosomal fraction (Fig. 1a). The 160 kDa band represents a crosslink between Ssb1 and the cytosolic Sse1, which functions as a cochaperone of the cytosolic pool of Ssb (refs 35, 36, 37) (Fig. 1a; Supplementary Fig. 1d). Many of the weaker crosslinks migrated with a molecular mass compatible with crosslink products between Ssb and ribosomal proteins, most of which possess a molecular mass between 6 and 25 kDa. Consistently, most of the smaller crosslinks were recovered in the ribosomal pellet (Fig. 1a, green asterisks). We next probed the crosslinks obtained in a wild type or in a Δssb1Δssb2 extract with antibodies directed against ribosomal proteins surrounding the tunnel exit (Fig. 1b). While no differences in the crosslinking pattern of Rpl25, Rpl17, Rpl26 or Rpl31 between the Ssb1* and Δssb1Δssb2 strains were observed, Rpl35 (13.9 kDa), Rpl39 (6.3 kDa) and Rpl19 (21.7 kDa) formed crosslink products of the expected molecular mass in the wild type but not in a Δssb1Δssb2 extract (Fig. 1c). The general crosslinking pattern observed in extracts derived from wild type cells was similar, with bands of slightly weaker intensity, than observed in extracts from the Ssb1* strain (Supplementary Fig. 1e). The crosslink band detected with αRpl35 was fuzzy or, in some experiments, migrated as a doublet of bands (Fig. 1c), which both represented crosslinks between Ssb and Rpl35 (Supplementary Fig. 1f). From these data, we conclude that Ssb contacts Rpl35, Rpl39 and Rpl19, which are all located in close proximity of the tunnel exit.
(a) Ssb is crosslinked to proteins of the large ribosomal subunit. Isolated ribosomes of the Ssb1* strain were incubated with (+) or without (−) the crosslinker BS3. The crosslinked sample (Total) was separated into a cytosolic supernatant (cyt) and a ribosomal pellet (ribo) via centrifugation through a low-salt sucrose cushion and was analysed via immunoblotting with αSsb. Shown is a short exposure of the upper part of the blot (>116 kDa), which contains the strong crosslink between Ssb1* and Sse1 (Ssb1*–Sse1) and a long exposure of the lower part of the blot (<116 kDa), which contains the weaker crosslinks between Ssb1* and ribosomal proteins (green asterisks). Rpl17 served as a marker for the ribosomal fraction. (b) Schematic representation of selected ribosomal proteins (Rpl19, Rpl35 and Rpl39 in pink; Rpl31 in blue; Rpl17, Rpl25 and Rpl26 in white) surrounding the tunnel exit (black) of the yeast ribosomal large subunit (grey). (c) Identification of ribosomal proteins crosslinked to Ssb1*. Crosslinking was performed in total cell extract of Ssb1* or Δssb1Δssb2 strains. Aliquots were analysed via immunoblotting using αSsb, αRpl35, αRpl39, αRpl25, αRpl19, αRpl17, αRpl26 and αRpl31 as indicated. Crosslink products between Ssb1* and ribosomal proteins (Ssb1*-XL) are indicated with red asterisks.
Structure determination employing a cysteine-crosslink
Having identified the Ssb interacting ribosomal proteins, we wanted to understand the molecular details of the Ssb ribosome interactions. Therefore, we set out to determine the crystal structure of Ssb from the thermophilic fungus Chaetomium thermophilum33,34, which is 75% identical to Ssb1 from Saccharomyces cerevisiae. Within the Hsp70 family, domain organization is highly conserved (Fig. 2a; Supplementary Fig. 2; Ssb residue numbering is given for C. thermophilum unless stated otherwise). In order to crystallize full-length Ssb, we stabilized the ATP-bound, open conformation adapting a cysteine crosslinking strategy that was successfully used for the structure determination of Escherichia coli DnaK (ref. 11). To identify the relevant residues to create the cysteine bridge, we created a homology model of full-length Ssb based on DnaK (refs 11, 38) using SWISS-MODEL39,40. On the basis of this model, the conserved threonine in the NBD P-loop was replaced by an alanine (T208A) to reduce the intrinsic ATPase activity. Two cysteines (in the NBD and SBDα) had to be introduced to link both domains via a disulfide bond. While the position in the NBD was readily determined (E51), we could not directly identify the relevant position of the second cysteine from the homology model. Therefore seven residues (534–540) in Ssb SBDα were individually mutated. After purification and oxidation, only the Ssb T208A-E51C-D534C mutant crystallized in the presence of ATP (Supplementary Fig. 3a,b). The crystals belong to the monoclinic space group P 1 21 1 with unit cell parameters of a=66.9 Å, b=128.2 Å and c=79.7 Å (Table 1) and contain two molecules in the asymmetric unit. The structure was solved at 2.6 Å resolution by molecular replacement using DnaK as a search model11 (Table 1). The resulting electron density map was of high quality showing well defined ATP and Mg2+ ligands, as well as the engineered C51–C534 disulfide bridge (Supplementary Fig. 4). The final model has excellent stereochemistry (Ramachandran allowed 99.5%) and only small parts of the structure are disordered (residues 1–3, 393–394, 561–571 and 613–614), indicated as dotted lines (Fig. 2b).
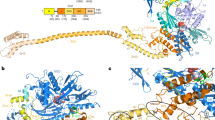
(a) Domain architecture of Ssb (residue numbers are given for C. thermophilum; corresponding residues in S. cerevisiae are in brackets). Ssb comprises an NBD (shades of blue) and an SBD consisting SBDβ (purple) and SBDα (lid domain, brown). A conserved linker (DLLLLDV, green) connects the NBD to the SBD. (b) Crystal structure of Ssb in the open conformation (cartoon representation). Ssb is a canonical Hsp70 with the NBD (subdomains IA, IIA, IB and IIB in different shades of blue) contacting both the SBDβ and the SBDα. SBDα is fixed on the NBD (bound ATP shown in sticks) by an engineered disulfide bridge (shown as sticks, see Supplementary Fig. 4). (c) Surface representation of Ssb (left and middle panel) and DnaK (right panel; pdb code 4b9q (ref. 11)) coloured by residue conservation (left panel) or electrostatic surface potential (middle and right panel). Insets show a close-up view of conserved residues from Ssb-SBDα forming a positively charged surface patch absent in DnaK. Surface charge spans from −4 kT/e (deep red) to +4 kT/e (deep blue). Conservation surface mapping are as reported by ConSurf84 and spans from variable (cyan) to conserved (deep red). Figures were produced using PDB2PQR (refs 71, 72) and APBS (ref. 70) in PyMOL (ref. 68).
Crystal structure of ATP-bound Ssb
Ssb is a typical member of the Hsp70 family and the crystal structure shows the canonical domain architecture (NBD, SBDβ and SBDα) of Hsp70 chaperones (Fig. 2a,b). The Ssb nucleotide binding domain (NBD, blue) and the substrate binding domain (SBD, purple and brown) are connected by the conserved inter-domain linker DLLLLDV (green) responsible for allosteric communication15,41.
The Ssb NBD, the β-sheet and α-helical subdomains of the SBD are arranged as in the open (ATP-bound) conformation of DnaK (ref. 11). The NBD (residues 4–392) has the typical actin-like fold42 and comprises two lobes (I and II), further divided into four subdomains (IA, IB, IIA and IIB) (Fig. 2b). ATP binds between the two lobes. Aspartate 13 coordinates the Mg2+ ion through two water molecules, and Mg2+ in turn coordinates both the ATP β and γ phosphates (Supplementary Figs 4a and 5a). Superposition of the NBDs (including the active site) of Ssb with DnaK shows an r.m.s.d. below 1 Å (for 358 Cα atoms) underlining the high structural conservation (Supplementary Fig. 5b).
As in the ATP-bound open conformation of DnaK, the β-sheet subdomain of the SBD (Ssb-SBDβ, residues 404–513) contacts the NBD subdomains IA, IIA and IB (Fig. 2b). It shows the typical, distorted β-sandwich fold formed by two layers of β-sheets built by three and five strands (Supplementary Fig. 5c,d). In Hsp70s the residues involved in substrate binding are conserved and the loop between the first and second β-strand of the SBDβ (L1–2) is important for substrate binding as it contributes to the shape of the binding cleft (Supplementary Fig. 2). When we compare Ssb-SBDβ with DnaK-SBDβ two important differences are observed: methionine 410 (threonine 403 in DnaK) seems to restrict the entrance to the substrate binding cleft, and glutamate 411 (methionine 404 in DnaK) may be engaged in polar contacts with the substrate. Methionine 404 in DnaK forms van der Waals contacts with the central leucine of the substrate peptide43 (Supplementary Fig. 5c,d). These adaptations in Ssb-SBDβ are conserved in all members of the Ssb subfamily (Supplementary Fig. 2) but their influence on Ssb substrate specificity has not been addressed so far.
In Ssb, the SBDβ is followed by SBDα (residues 517–613, helical lid domain) that comprises four α-helices (A–D), with αB to αD forming a three-helix bundle. Our structure shows a high degree of flexibility for this helical bundle as indicated by high B-factors (Supplementary Fig. 6). In cytosolic Hsp70 proteins SBDα comprises five α-helices. In Ssb, the helix αC of Ssb-SBDα contains a nuclear export signal (NES) (ref. 44) (residues 575IEQALSEAM583) of which the hydrophobic side chains are engaged in van der Waals contacts forming the hydrophobic core of the bundle.
The Ssb-SBDα is required for ribosome binding
Further analysis of the Ssb structure in combination with a detailed sequence alignment revealed the presence of a positive patch located in SBDα. This positive patch is mainly formed by conserved Arg and Lys residues (K597, K598, K604, R605 and K609) located in the helices αC and αD (Fig. 2c, left and middle panel; Fig. 3a). These residues are conserved in members of the Ssb subfamily (Fig. 2c, left panel) but not in canonical Hsp70s, as shown by comparison with DnaK (ref. 11) (Fig. 2c, middle and right panel). To test whether these Ssb specific, positively charged residues are involved in Ssb–ribosome interaction, we created a series of mutants, in which the relevant positively charged residues had been replaced pairwise and performed quantitative ribosome binding assays (Fig. 3). The first pair K568/R569 (Sc K567/R568) is in a disordered loop connecting helices αB and αC (Ssb LBC), the second and third pair K597/K598 (Sc R596/K597) and K604/R605 (Sc K603/R604) are located in helix αD (Ssb D1 and Ssb D2, respectively). We created reverse charge double mutants in yeast Ssb1, resulting in three variants: Ssb LBC (K567E/R568E), Ssb D1 (R596D/K597D) and Ssb D2 (K603D/R604D) (Fig. 3a). As the Ssb antibody is directed against the very C-terminus of Ssb, mutations within this region strongly affect antibody recognition (Supplementary Fig. 7a), therefore an N-terminally myc-tagged Ssb1 (mycSsb1) was employed in these experiments (Supplementary Fig. 7a). All three mutants (LBC, D1, and D2) fully complemented growth defects of a Δssb1Δssb2 strain (Fig. 3b). However, ribosome-binding of the three mutants was reduced to less than 50% of the mycSsb1 control (Fig. 3c,d; Supplementary Fig. 7b). Due to possible synergistic effects of these mutations, double (LBC–D1, LBC–D2) and triple (LBC–D1–D2) mutants were tested. While double mutants fully complemented growth defects of the Δssb1Δssb2 strain, the triple mutant displayed slightly reduced growth at 20 °C and at 30 °C in the presence of 50 μg ml−1 paromomycin (Fig. 3b). When the paromomycin concentration was raised to 500 μg ml−1, a concentration at which even growth of the wild type strain was reduced, the triple LBC–D1–D2 mutant displayed a severe growth defect (Fig. 3b). However, all three mutants were severely defective with respect to ribosome-binding (Fig. 3c,d). These findings indicated that the growth defect of the LBC–D1–D2 mutant was not due to a defect in ribosome binding, but was due to a negative effect caused probably by the accumulation of negative charges within the very C-terminal region of Ssb1. This is also supported by the observation that, consistent with previous data16, a series of Ssb1 C-terminal truncation variants, lacking the last three (ΔC3), eight (ΔC8) or twenty-three (ΔC23) residues fully supported growth of the Δssb1Δssb2 strain (Fig. 3b), but displayed severe ribosome-binding deficiency (Fig. 3c,d; Supplementary Fig. 7b) as did internal deletions (ΔNES) (Fig. 3b–d; Supplementary Fig. 7b). In order to assay the effect of these variants on the integrity of the helical bundle, we analysed them by CD spectroscopy. As expected from the crystal structure, the ΔNES variant showed reduced helicity compared with the reverse charge mutants and the WT (Supplementary Fig. 3c,d). These effects could be explained by destabilization of the helical bundle due to disruption of the hydrophobic core. Taken together, our data indicate that the loop LBC and helix αD as part of the three-helix bundle form an interaction platform that allows Ssb to efficiently bind to the ribosome. The interaction was strictly dependent on conserved, positively charged residues clustering into a three-dimensional epitope.
(a) Sequence alignment of the SBDα C-terminal part from Ssb family members (upper block) and canonical Hsp70s (lower block). Secondary structure elements based on the crystal structure of CtSsb and EcDnaK are depicted on top in blue (helix αC and αD) and bottom in yellow (helix αB, αC, αD, and αE), respectively. Representation generated with ESPRIPT74. Ct: Chaetomium thermophilum, Sc: Saccharomyces cerevisiae, Cg: Chaetomium globosum, An: Aspergillus nidulans, Sp: Schizosaccharomyces pombe, Hs: Homo sapiens, Ec: Escherichia coli. *: indicates the position of the A577K mutation within Ssb1 (Ssb1*). The mutations within the positive patches are indicated above the CtSsb sequence (LBC, D1 and D2). (b) Mutants within the C-terminal 50 residues of Ssb1 rescued the growth defects of a Δssb1Δssb2 strain. Serial 10-fold dilutions of strains expressing myc-tagged versions of Ssb1 were spotted onto YPD plates and were incubated as indicated. Paromomycin was added to plates as indicated. (c) Ssb ribosome-binding is affected by mutations in SBDα. Aliquots of cell extract (Total) and ribosomes isolated under low-salt conditions (Ribo) were analysed using αmyc, αSse1 and αRpl35. Sse1 and Rpl35 in total extract are shown as loading controls. A longer exposure of the ribosome fraction blot is shown to visualize the Ssb variants with weak ribosome binding. (d) Quantitative analysis of the ribosome-bound fraction of Ssb variants. Quantifications are based on at least three independent experiments (Supplementary Fig. 7b); error bars represent the s.d. Ribosome-binding of the mycSsb1-ΔC23 mutant was below the detection limit. Ssb LBC: K567E/R568E, Ssb D1: R596D/K597D and Ssb D2: K603D/R604D.
Ssb–ribosome interaction is modulated by RAC
Ssb1 interacts with the ribosome by contacting Rpl35 and Rpl39 in close proximity to the tunnel exit. The Ssb cochaperone RAC (refs 3, 14) contacts Rpl31 and stimulates ATP hydrolysis in Ssb (Fig. 1b, blue)29,30,31,32. Therefore, we wanted to test whether RAC influences Ssb binding to the ribosome. We employed a strain lacking RAC (Δzuo1Δssz1) or a strain where wild type Zuo1 was replaced by the Zuo1-H128Q variant (RAC-H128Q), which does not stimulate the ATPase activity of Ssb due to a mutation in the conserved HPD motif in the J-domain32. With respect to ribosome binding, RAC-H128Q behaves similar to wild type RAC, which fully binds to ribosomes under low-salt conditions and is released from ribosomes under high-salt conditions (Fig. 4a). However, ribosome binding of wild type Ssb1 was significantly reduced in a Δzuo1Δssz1 or in a RAC-H128Q strain (Fig. 4a,b; Supplementary Fig. 8) indicating that ribosome-association of Ssb was hampered when RAC was absent or non-functional as a cochaperone. We next probed the interaction of Ssb with Rpl35 and Rpl39 in the Δzuo1Δssz1 and RAC-H128Q strains employing the strong crosslinks of Ssb1 to Rpl35 and Rpl39 in the wild type strain (Figs 1c and 4c). The crosslink between Ssb1 and Rpl35 was reduced to less than 10% and the crosslink between Ssb1 and Rpl39 was reduced to less than 5% when RAC was absent or non-functional (Fig. 4c–e; Supplementary Fig. 8). These results suggested that RAC-stimulated ATP hydrolysis in Ssb, introducing the ADP-bound (closed) conformation, modulated the interaction of Ssb with Rpl35 and Rpl39. We therefore employed the Ssb1-K73A mutant, which is unable to hydrolyze ATP and thus does not complement growth defects of a Δssb1Δssb2 strain23. Ssb1-K73A also showed moderately reduced binding to ribosomes, similar to the ribosome-binding defect observed in the absence of functional RAC (Fig. 4a,b). Importantly, however, crosslinking of Ssb1-K73A to Rpl35 and Rpl39 was severely reduced (Fig. 4d,e). Thus, the combined data strongly support a model in that in the closed conformation Ssb was in close contact to Rpl35 and Rpl39, while this was not the case in the open conformation.
(a) Ribosome-binding of Ssb is reduced when RAC is absent, non-functional, or if ATP hydrolysis is prevented by the Ssb1-K73A mutation. Total cell extract (tot) was separated into a cytosolic fraction (cyt) and a ribosomal pellet (ribo) under low-salt (LS) or high-salt (HS) conditions. Immunoblots were decorated with αSsb, αZuo1, αSse1 (cytosolic marker) or αRpl35 (ribosomal marker). (b) The ribosome-bound fraction of Ssb in extracts derived from RAC-H128Q, Δzuo1Δssz1 or Ssb1-K73A strains is reduced. Quantification of the ribosome-bound fraction of Ssb under low-salt conditions is based at least on 3 independent experiments (Supplementary Fig. 8), error bars represent the s.d. (c,d) The contact between Ssb1 and Rpl35 or Rpl39 is strongly reduced if RAC is absent, non-functional, or if ATP hydrolysis is prevented by the Ssb1-K73A mutation. Crosslinking was performed in cell extracts of strains expressing Ssb1* (Supplementary Table 1) and (c) wild type RAC, RAC-H128Q, or carrying the Δzuo1Δssz1 mutation or (d) carrying the Ssb1*-K73A mutation. Immunoblots were decorated with antibodies directed against Rpl35 or Rpl39. Crosslink products between Ssb1* and ribosomal proteins (Ssb1*-XL) are indicated with red asterisks. (e) Comparison of crosslinking efficiencies between Ssb1* and Rpl35 or Rpl39 in cell extracts derived from Ssb1* strains expressing wild type RAC, RAC-H128Q, carrying the Δzuo1Δssz1 mutation, or expressing Ssb1*-K73A. The band intensities of crosslink products between Ssb1* and Rpl35 (Ssb1*-Rpl35) or Rpl39 (Ssb1*-Rpl39) were determined in at least 3 independent experiments. The intensity of Ssb1*-Rpl35 and Ssb1*-Rpl39 crosslinks in the Ssb1* strain was set to 100%. Error bars represent the s.d. (f) CRAC analysis on ProtA-TEV-His6-Ssb1 (red) and negative control (black). The number of hits representing the number of times a nucleotide was mapped onto the 37S rDNA reference sequence of S. cerevisiae is shown. (g) Schematic representation of the expansion segments ES41, ES24 and ES39 of the 25S rRNA crosslinked to Ssb1 (ref. 85). Nucleotides found in all hits are highlighted and nucleotides representing the nucleotides frequently mutated or deleted in the CRAC experiments are highlighted in dark colour.
Ssb contacts specific expansion segments of rRNA
As the interaction of Ssb with nontranslating ribosomes is salt sensitive3,18 (Fig. 4a) and we detected a strict requirement of a positive surface patch for ribosome binding, we anticipated that ribosome binding of Ssb might involve interaction with ribosomal RNA. To experimentally test this hypothesis, we used the CRAC (ultraviolet crosslinking and analysis of complementary DNA (cDNA)) methodology45, which was previously used successfully to identify RNA-protein interactions in ribosomal subunits45,46,47. We found that Ssb1 directly contacts rRNA elements on the 60S subunit. Ssb1 was efficiently crosslinked to the eukaryote specific expansion segments ES24 and ES41 close to the tunnel exit and to ES39 more distant from the tunnel exit (Fig. 4f,g). These data support the contribution of the positively charged patch on Ssb to the interaction with rRNA.
Discussion
Ssb interacts with nascent polypeptide chains when they emerge from the ribosomal tunnel exit3,18,26 and so it was long anticipated that Ssb must interact with the ribosome close to the polypeptide tunnel exit. However, the exact localization, molecular details of this interaction, and the interplay with its cochaperone RAC remained unclear.
The crystal structure in the open ATP-bound conformation shows that Ssb represents a canonical member of the Hsp70 family. Overall, the structure is highly similar to the E. coli Hsp70 homologue DnaK in its open conformation11,48. The structure was readily obtained using a crosslinking approach developed for DnaK (ref. 11) underlining the high conservation within the Hsp70 family. The nucleotide and substrate binding domains are connected by a conserved, hydrophobic linker region. The substrate binding site is more narrow compared with DnaK due to specific changes in SBDβ, probably reflecting different substrate specificity. The Ssb SBDα comprises four helices and displays high flexibility. All elements required for ATP binding, for substrate binding and for allosteric regulation are present, suggesting that Ssb follows the same molecular mechanism of allostery as DnaK (ref. 15).
Ssb SBDα exposes a positively charged surface region conserved in all members of the Ssb subfamily of Hsp70s, but not in canonical cytosolic Hsp70s such as Ssa. Comparison with the closed conformation of DnaK shows that also in the closed conformation of Ssb this region would be solvent exposed and contacts neither SBDβ nor the NBD, indicating that it is available to interact with another partner. To address if the positively charged patch is involved in the interaction of Ssb with ribosomes, we performed crosslinking experiments in combination with ribosome binding assays. A combination of structure based in vivo and in vitro mutational analysis of three positive patches within the SBDα lid domain demonstrated the importance of these residues for ribosome binding. Although these mutations impaired, or when combined, abolished ribosome binding of Ssb almost entirely, cell growth was not significantly affected. This observation suggests that the direct interaction of Ssb with ribosomes is less important than previously anticipated. However, the finding is not too surprising if one considers that Ssb-type Hsp70s are confined to Ascomycota and, for example, mammalian cells do not contain Ssb-type Hsp70 homologues, which directly interact with ribosomes49. Likely, when Ssb is not prepositioned on the ribosome, Ssa, the other major cytosolic Hsp70 in yeast, contributes to cotranslational protein folding and thereby compensates for the ribosome binding defects of the Ssb lid domain mutants28. Indeed, Ssa, together with the J-domain chaperone Jjj1 can assist cotranslational protein folding in yeast50.
Using CRAC methodology we identified three rRNA expansion segments (ES24, ES39 and ES41) on the 60S ribosomal subunit as direct interaction partners of Ssb. While ES24 and ES41 are located close to the tunnel exit (Fig. 5), ES39 is more distant. In the presence of RAC, ES39 appears shielded while ES41 and ES24 seem available for Ssb binding30,31. The crosslink to ES39 could therefore indicate an additional rRNA binding site of Ssb when RAC is absent. Using ribosome-binding experiments we also detected residue-specific contacts between SBDα and the ribosome. Our data indicate that Ssb interacts with Rpl35, Rpl39 and Rpl19 close to the tunnel exit. Using the crosslink data as restraints, we positioned the structure of ATP-bound Ssb on the ribosome51. SBDβ was docked close to the exit tunnel to allow for the interaction with a nascent chain and SBDα was oriented towards ES41. Choosing this orientation, SBDα is wedged in between Rpl22 and Rpl31, and within crosslinking distance to ES24 (Fig. 5a, Supplementary Fig. 9). While this orientation of the ATP-bound Ssb in the open conformation correlates with the crosslinks to ES41 and ES24, SBDα is not in close proximity of Rpl35 and Rpl39. We therefore created a model of Ssb in the closed conformation (based on DnaK (ref. 43)) as switching between the open and closed states relocates SBDα by >60 Å (Fig. 5b). When we docked a model of ADP-bound Ssb (closed conformation) via the SBDβ to the tunnel exit as before, SBDα is in close proximity of Rpl35 and Rpl39. Thus, docking Ssb to the ribosome in the open or closed conformation faithfully correlates with the observed protein and rRNA crosslinks and indicates that the structural rearrangements underlying allosteric regulation of Hsp70 proteins should also occur in Ssb and give rise to specific crosslinks. This model is further supported by the observation that the crosslinks to Rpl35 and Rpl39 were significantly reduced when Ssb cannot hydrolyze ATP (K73A mutant), as well as when RAC was absent or non-functional. Because RAC is strictly required for efficient ATP hydrolysis in Ssb (ref. 32), these data indicate that crosslinking to Rpl35 and Rpl39 was indeed confined to the closed (ADP-bound) conformation of Ssb. Consistently, it was previously shown that Ssb fails to interact with nascent chains in the absence of functional RAC (ref. 26), as the high affinity substrate binding state of Ssb is not induced. In summary, Ssb interacts with the 60S ribosomal subunit in a dual manner: by specific protein-protein as well as protein-rRNA contacts. Both types of interaction require the Ssb lid domain and are modulated by the cochaperone RAC.
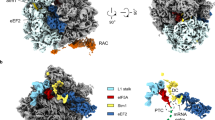
(a) Ssb in the open conformation (ATP-bound, pre-hydrolysis state) binds close to the tunnel exit. The model is based on a molecular model of Ssb at the ribosomal tunnel exit (shown in Supplementary Fig. 9). In this state, the substrate (nascent chain, NC; purple) is not tightly bound (low affinity state). (b) On interaction with the cochaperone ribosome-associated complex (RAC), ATP is hydrolyzed and Ssb switches to the closed conformation (ADP-bound, post-hydrolysis state), which involves flipping of SBDα onto the SBDβ. Now Ssb can tightly interact with the nascent chain via SBD (high affinity state). The ribosome is shown in grey, the 60 S and 40 S subunits are indicated. Exposed expansion segments of rRNA (ES41, ES24) and ribosomal proteins (Rpl19, Rpl22, Rpl31, Rpl35, Rpl39) are depicted in orange and salmon, respectively. The tunnel exit is highlighted by a black circle (exit). Ssb is shown in blue and domains are labelled (NBD, SBDα, SBDβ).
The ribosomal surface around the tunnel exit, which serves as a binding platform for RAC and Ssb, has been described as an universal adaptor site for cotranslational factors including chaperones, enzymes and targeting factors2,52,53. It provides two main binding sites: Rpl25/Rpl35 (L23/L29 in E. coli) for SRP, trigger factor, the translocon, YidC/Oxa1 and Map and Rpl31/Rpl17 (L17/L22 in eubacteria) for RAC, the nascent chain associated complex (NAC), the SRP receptor and peptide deformylase. The crosslinks of Ssb to ES41, which is next to the Rpl31/Rpl17 site and to Rpl35, which is part of the other site, indicate that Ssb bridges both binding sites. Interestingly, during ribosome biogenesis control mechanisms are implemented to ensure that the universal adaptor site is not only correctly assembled, but also protected from premature, unproductive interactions54. The ribosomal surface around the tunnel exit directs the highly dynamic interplay of a myriad of factors and we only begin to understand these complex interaction networks.
Methods
Strains and plasmids
Strains and plasmids are listed in Supplementary Table 1 and Supplementary Table 2. The nomenclature for ribosomal proteins used in this study is provided in Supplementary Table 3.
DNA cloning and plasmid preparation were performed according to standard methods55. All constructs for the expression of mutants and tagged proteins were verified by sequencing and Western blot analysis.
The Chaetomium thermophilum (Ct) SSB coding sequence fused to an N-terminal His6 tag was PCR amplified from the Ct cDNA and cloned into pET24. The mutations T208A, E51C and D534C were introduced by site-directed mutagenesis as described by the manufacturer (QuikChange Lightning, Agilent Technologies). The coding sequence of the 3-helical bundle of Ssb (residues 536–614) fused to an N-terminal His6 tag was amplified by PCR from pET24a-His6Ssb-E51C-T208A-D534C and cloned into the pET24a vector. Mutations LBC (K568E/R569E), D1 (K597D/K598D), D2 (K604D/R605D), ΔNES (Δ575-587) or a combination of them were introduced by site-directed mutagenesis.
The Saccharomyces cerevisiae (Sc) strains MH272-3f and BY4742 are the parental wild type strains of all haploid strains used in this study. MH272-3f strains lacking SSB1 and SSB2 (Δssb1Δssb2), ZUO1 (Δzuo1), SSZ1 (Δssz1) or lacking combinations of these genes were described previously22,23,56. The strain lacking SSB1, SSB2 and ZUO1 (Δssb1Δssb2Δzuo1) was generated by mating Δzuo1Δssz1 (ref. 22) with Δssb1Δssb2 (ref. 23) followed by dissection and tetrade analysis. The SSB1 open reading frame +/−300 bp up- and down-stream was cloned into pYCPlac33 (ref. 57). The resulting plasmid pYCPlac33-Ssb1 was used as a template to generate a single alanine to lysine exchange at position 577 of Ssb1 via QuikChange resulting in the low copy plasmid pYCPlac33-Ssb1*. SSB1* ±300 bp was transferred into the high copy plasmid pYCPlac195 resulting into pYCPlac195-Ssb1* (ref. 57). The Ssb1-K73A mutant contains an amino acid exchange within the ATPase domain of Ssb1, which prevents ATP hydrolysis23,58. To generate Ssb1*-K73A, the PstI/BglII fragment in pYCPlac33-Ssb1-K73A was replaced by the PstI/BglII from pYCPlac33-Ssb1-A577K. To obtain N-terminally myc-tagged (MEQKLISEEDL) versions of Ssb1, the SSB1 open reading frame was cloned into pCM190 (Euroscarf) resulting in pCM190-mycSsb1. Mutations within the Ssb1 SBDα were introduced by PCR using pCM190-mycSsb1 as a template. Deletion of residues 574–586 within Ssb1 resulted in pCM190-mycSsb1-ΔNES. Deletion of the C-terminal residues SSR (ΔC3), VTKAMSSR (ΔC8) and SADELRKAEVGLKRVVTKAMSSR (ΔC23) resulted in pCM190-mycSsb1-ΔC3, pCM190-mycSsb1-ΔC8 and pCM190-mycSsb-ΔC23, respectively. All pCM190-derived mycSsb expression plasmids were transformed into the Δssb1Δssb2 strain. Zuo1-H128Q (ref. 26) was subcloned into pYCPlac181 (ref. 57) resulting in pYCPlac181-Zuo1-H128Q.
For CRAC analysis, the integration of the ProtA-TEV-His6 tag at the N-terminus of the SSB1 coding sequence into the BY4742 genome was performed as described59,60. In brief, a cassette containing the clonNAT resistance gene linked to a 390 nucleotides region upstream of SSB1 coding sequence fused to the ProtA-TEV-His6 tag sequence was PCR amplified using forward S1 and reverse S4 primers59,60 and the purified PCR product was transformed into BY4742. The transformants were selected on YPD (1% yeast extract, 2% peptone and 2% dextrose) supplemented with 100 μg ml−1 clonNAT agar plates.
Protein preparation and crystallization
The His6Ssb-E51C-T208A-D534C variant was produced in E. coli BL21(DE3) Rosetta2 cells. Lysogeny Broth (LB) media cultures supplemented with 1.75% (w/v) lactose, kanamycin (50 μg ml−1) and chloramphenicol (34 μg ml−1) were grown for 18 h at 30 °C. Cells were harvested and resuspended in lysis buffer (20 mM Hepes/NaOH pH 8, 250 mM NaCl, 20 mM MgCl2, 20 mM KCl), lysed in a microfluidizer (M1-10 L, Microfluidics) and purified by IMAC (Immobilized metal ion affinity chromatography, HisTrap HP 1 ml, GE Healthcare): after application of the lysate, the column was washed with 20 column volumes of lysis buffer supplemented with 20 mM imidazole and was eluted with 5 column volumes of lysis buffer supplemented with 200 mM imidazole. The eluate was diluted 10 fold with ATP binding buffer (20 mM Hepes/NaOH pH 7.5, 20 mM NaCl, 5 mM MgCl2, 0.1 mM EGTA (ethylene glycol-bis(β-aminoether)-N,N,N′,N′-tetraacetic acid), 1 mM DTT (1,4-Dithiothreitol), 10% (v/v) glycerol) and was applied to an agarose column with immobilized N6-(6-Amino)hexyl-ATP (Jena Biosciences). The column was washed with 20 column volumes of ATP binding buffer supplemented with 500 mM NaCl and eluted with 10 column volumes of ATP binding buffer supplemented with 5 mM ATP. The eluate was diluted 10-fold with ATP binding buffer containing 5 mM ATP but lacking DTT and concentrated 10-fold using centrifugal filter units (Merck Millipore).
The oxidation of the disulfide bond (C51–C534) was catalysed by the addition of a CuSO4 and 1,10-Phenanthroline solution with a final concentration of 0.5 mM and 1.75 mM, respectively, and incubation for 30 min at room temperature. To minimize the formation of inter-molecular disulfide bonds, the concentration of the protein was kept below 10 μM. The oxidized Ssb was further purified by size exclusion chromatography (SEC; S200-26/60, GE Healthcare) in SEC buffer (20 mM Hepes/NaOH pH 7.5, 150 mM NaCl, 5 mM MgCl2, 10 mM KCl, 10% (v/v) glycerol).
Crystallization screens were performed at 291 K by the sitting-drop vapor-diffusion method upon mixing equal volumes (0.2 μl) of the Ssb protein solution (10 mg ml−1) and reservoir solution containing 20% (v/v) PEG 3350 and 0.2 M NH4H2PO4. Crystals grew after 3 days.
The three-helix bundle of Ssb and variants were expressed in E. coli BL21(DE3) Rosetta2 cells grown in LB media supplemented with kanamycin and chloramphenicol. Overexpression was induced with isopropyl-1-thio-β-D-galactopyranoside (IPTG) at an OD600 of 0.8–1.0 and the culture incubated overnight at 18 °C. Cells were harvested and resuspended in lysis buffer (20 mM Hepes/NaOH pH 7.5, 300 mM NaCl, 20 mM MgCl2, 20 mM KCl), lysed in a microfluidizer and purified by IMAC: after application of the lysate, the column was washed with 20 column volumes of lysis buffer supplemented with 20 mM imidazole and eluted with 5 column volumes of lysis buffer supplemented with 200 mM imidazole. The eluate was applied to a SEC column S200 26/60 (size exclusion chromatography, GE Healthcare) run in SEC buffer (20 mM Hepes/NaOH pH 7.5, 300 mM NaCl, 5 mM MgCl2, 10 mM KCl). Samples were dialyzed overnight at 4 °C against CD buffer (10 mM KH2PO4/K2HPO4 pH 7.5, 150 mM KF).
Data collection and structure determination
Crystals were flash-frozen in liquid nitrogen after cryo-protection by transfer into cryo-solution containing mother liquor and 20% (v/v) glycerol. Diffraction data were measured under cryogenic conditions (100 K; Oxford Cryosystems Cryostream) at the European Synchrotron Radiation Facility (ESRF, Grenoble). Data were processed with XDS (ref. 61). Phases were obtained by molecular replacement using PHASER (ref. 62) (search model 4b9q11) in the PHENIX software package63. Model building, refinement and validation were done using COOT (ref. 64), Phenix.refine65, REFMAC5 (ref. 66) and MOLPROBITY67, respectively. Figures were prepared in PyMOL (ref. 68) and UCSF-Chimera69. Electrostatic surface potentials were calculated with APBS and PDB2PQR (refs 70, 71, 72) integrated in PyMOL (ref. 68). Sequence alignments were performed using Clustal Omega73 and visualized with ESPript 3.0 (ref. 74) ( http://espript.ibcp.fr/ESPript/ESPript/). Protein sequence conservations were analysed using the ConSURF server75,76.
Phenotypic assays
Growth defects were analysed by spotting 10-fold serial dilutions containing the same number of cells onto YPD or YPD+25, 50 or 500 μg ml−1 paromomycin agar plates, and were incubated for 2 days at 30 °C or 37 °C or 3 days at 20 °C, as indicated.
CRAC analysis
In vivo CRAC experiments were performed using the BY4742 ProtA-TEV-His6-Ssb1 and the parental BY4742 strain as negative control. Yeast cultures were grown to an OD600 of 2.0 in YPD, harvested and suspended in PBS (phosphate-buffered saline) before ultraviolet-irradiation in a Megatron UV chamber (1.6 J cm−2) for 3 min. The RNA crosslinked to the protein of interest was treated as described45,47 with the omission of transfer to nitrocellulose. Bands corresponding to the size of the protein of interest and higher were excised directly from the Bis-Tris NuPAGE gel (4–12%, Novex) and were subsequently digested by proteinase K. Extracted RNAs were amplified by RT-PCR. The obtained cDNAs were sequenced using the Illumina HiSeq sequencing platform and the reads were treated and analysed as described77 with one modification: the reads were mapped to S. cerevisiae genomic reference sequence using Bowtie 2 (v 2.2.5) (ref. 78). Two independent experiments were performed for each sample and one representative experiment is shown.
Crosslinking assays
Protein crosslinking reactions were performed with the homobifunctional, amino-reactive crosslinker BS3 (bis-(sulfosuccinimidyl)-suberate, spacer length 1.14 nm, Thermo Scientific) either with isolated ribosomes29 or with total cell extracts prepared from freshly harvested yeast cells, rapidly frozen in liquid nitrogen, and subsequently disrupted with a cryo-mill (MM400, Retsch)79. About 500 μl of the cyro-mill cell powder (stored at −80 °C) was then resuspended in 1,200 μl crosslinking buffer (20 mM Hepes/KOH pH 7.4, 80 mM K(H3CCOO), 2 mM Mg(H3CCOO)2, 1 mM PMSF (phenylmethylsulfonyl fluoride), 2 mM DTT), cell debris were removed by centrifugation at 20,000 g for 2 min at 4 °C, and aliquots of the supernatant were then incubated in the absence or in the presence of 1.2 mM BS3 for 20 min at 21 °C. Crosslinking reactions were quenched by the addition of glycyl-glycine to a final concentration of 30 mM. Ribosomes and crosslinks to ribosomal proteins were collected by centrifugation at 180,000 g for 35 min. Ribosomal pellets were resuspended in 100 μl ribosome-binding buffer and were precipitated by addition of 5% trichloroacetic acid (TCA). TCA pellets were analysed on 10% Tris-Tricine gels followed by immunoblotting.
Ribosome binding assays
Yeast strains were grown to early log phase on YPD, cycloheximide was added to a final concentration of 100 μg ml−1, and subsequently cells were harvested via centrifugation at 4,500 g. Cell pellets were resuspended in ribosome-binding buffer (20 mM Hepes/KOH pH 7.4, 2 mM Mg(H3CCOO)2, 120 mM K(H3CCOO), 100 μg ml−1 cycloheximide, 2 mM DTT, 1 mM PMSF, protease inhibitor mix: 1.25 μg ml−1 leupeptin, 0.75 μg ml−1 antipain, 0.25 μg ml−1 chymostatin, 0.25 μg ml−1 elastinal, 5 μg ml−1 pepstatin A) and disrupted using the glass beads method80. After a clearing spin at 20,000 g, each 60 μl of the total glass beads extract (A260 between 40 and 250) was loaded onto a 90 μl low-salt sucrose cushion (25% (w/v) sucrose, 20 mM Hepes/KOH pH 7.4, 120 mM K(H3CCOO), 2 mM Mg(H3CCOO)2, 2 mM DTT, 1 mM PMSF, protease inhibitor mix) or onto a 90 μl high-salt sucrose cushion (25% (w/v) sucrose, 20 mM Hepes/KOH, pH 7.4, 600 mM K(H3CCOO), 2 mM Mg(H3CCOO)2, 2 mM DTT, 1 mM PMSF, protease inhibitor mix). After centrifugation at 400,000 g at 4 °C for 25 min the cytosolic supernatant was collected and the ribosomal pellet was resuspended in 100 μl ribosome-binding buffer. Aliquots of the total glass beads extract, cytosolic supernatant, and resuspended ribosomal pellets were precipitated by addition of 5% TCA. TCA pellets were analysed on 10% Tris-Tricine gels followed by immunoblotting. For quantification purposes, the loading of cytosolic supernatant and ribosomal pellet was adjusted such, that the intensities of both bands on immunoblots were in the linear range of the analysis (Supplementary methods). Quantification was performed using AIDA ImageAnalyzer (Raytest). For statistical analysis at least 3 independent experiments were examined.
Circular dichroism spectroscopy
Sample concentration was measured by Bradford assay against a BSA standard (Rotiquant Bradford reagent, Roth) and adjusted to 20 μM. The CD spectroscopy measurements were performed at room temperature in a ultraviolet-spectropolarimeter J750 (Jasco) using 1 mm quartz cuvettes (Hellma).
Immunoblotting
Proteins were separated on 10% Tris-Tricine gels. The following polyclonal antibodies were raised against peptides or purified proteins in rabbit: αSsb (ref. 19) (dilution 1:5,000), αRpl17 (ref. 19; dilution 1:20,000), αRpl24 (ref. 19; dilution 1:20,000) αRpl35 (dilution 1:20,000), αRpl39 (ref. 81; dilution 1:10,000), αRpl25 (ref. 82; dilution 1:10,000), αRpl19 (dilution 1:1,000), αRpl26 (dilution 1:4,000), αRpl31 (ref. 82; dilution 1:2,000), αSse1 (ref. 83; dilution 1:10,000) and αZuo1 (ref. 19; dilution 1:10,000) from EUROGENTEC (Bel S.A.). Monoclonal mouse αHis-tag (dilution 1:20,000, AbDSerotec, catalogue number: MCA1396) and αmyc-tag (dilution 1:5,000, Chemicon, catalogue number: CBL430) antibodies were used as indicated. Immunoblots were developed using ECL with horse-radish peroxidase-conjugated goat α-rabbit IgG (dilution 1:10,000, Pierce, catalogue number: 31460) or α-mouse (dilution 1:5,000, Santa Cruz Biotechnology, catalogue number: Sc-2005) as secondary antibody. Uncropped blots are shown in Supplementary Figs 10–29. Validation of previously unpublished antibodies is shown in Supplementary Fig. 30.
Data availability
Coordinates and structure factors have been deposited with the Protein Data Bank (PDB) under the accession codes: 5TKY. The data that support the findings of this study are available from the corresponding authors on request.
Additional information
How to cite this article: Gumiero, A. et al. Interaction of the cotranslational Hsp70 Ssb with ribosomal proteins and rRNA depends on its lid domain. Nat. Commun. 7, 13563 doi: 10.1038/ncomms13563 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Preissler, S. & Deuerling, E. Ribosome-associated chaperones as key players in proteostasis. Trends. Biochem. Sci. 37, 274–283 (2012).
Kramer, G., Boehringer, D., Ban, N. & Bukau, B. The ribosome as a platform for co-translational processing, folding and targeting of newly synthesized proteins. Nat. Struct. Mol. Biol. 16, 589–597 (2009).
Peisker, K., Chiabudini, M. & Rospert, S. The ribosome-bound Hsp70 homolog Ssb of Saccharomyces cerevisiae. Biochim. Biophys. Acta 1803, 662–672 (2010).
Giglione, C., Fieulaine, S. & Meinnel, T. N-terminal protein modifications: bringing back into play the ribosome. Biochimie 114, 134–146 (2015).
Dörfel, M. J. & Lyon, G. J. The biological functions of Naa10 - From amino-terminal acetylation to human disease. Gene 567, 103–131 (2015).
Grudnik, P., Bange, G. & Sinning, I. Protein targeting by the signal recognition particle. Biol. Chem. 390, 775–782 (2009).
Cross, B. C., Sinning, I., Luirink, J. & High, S. Delivering proteins for export from the cytosol. Nat. Rev. Mol. Cell Biol. 10, 255–264 (2009).
Wild, K. & Sinning, I. RNA gymnastics in mammalian signal recognition particle assembly. RNA Biol. 11, 1330–1334 (2014).
Mayer, M. P. & Bukau, B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol. Life Sci. 62, 670–684 (2005).
Mayer, M. P. Hsp70 chaperone dynamics and molecular mechanism. Trends Biochem. Sci. 38, 507–514 (2013).
Kityk, R., Kopp, J., Sinning, I. & Mayer, M. P. Structure and dynamics of the ATP-bound open conformation of Hsp70 chaperones. Mol. Cell 48, 863–874 (2012).
Bertelsen, E. B., Chang, L., Gestwicki, J. E. & Zuiderweg, E. R. Solution conformation of wild-type E. coli Hsp70 (DnaK) chaperone complexed with ADP and substrate. Proc. Natl Acad. Sci. USA 106, 8471–8476 (2009).
Zuiderweg, E. R. et al. Allostery in the Hsp70 chaperone proteins. Top. Curr. Chem. 328, 99–153 (2013).
Kampinga, H. H. & Craig, E. A. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat. Rev. Mol. Cell Biol. 11, 579–592 (2010).
Mayer, M. P. & Kityk, R. Insights into the molecular mechanism of allostery in Hsp70s. Front. Mol. Biosci. 2, 58 (2015).
Pfund, C., Huang, P., Lopez-Hoyo, N. & Craig, E. A. Divergent functional properties of the ribosome-associated molecular chaperone Ssb compared with other Hsp70s. Mol. Biol. Cell 12, 3773–3782 (2001).
Nelson, R. J. et al. The translation machinery and 70 KDa heat shock protein cooperate in protein synthesis. Cell 71, 97–105 (1992).
Pfund, C. et al. The molecular chaperone Ssb from Saccharomyces cerevisiae is a component of the ribosome-nascent chain complex. EMBO J. 17, 3981–3989 (1998).
Raue, U., Oellerer, S. & Rospert, S. Association of protein biogenesis factors at the yeast ribosomal tunnel exit is affected by the translational status and nascent polypeptide sequence. J. Biol. Chem. 282, 7809–7816 (2007).
Hübscher, V. et al. The Hsp70 homolog Ssb and the 14-3-3 protein Bmh1 jointly regulate transcription of glucose repressed genes in Saccharomyces cerevisiae. Nucleic Acids Res. 44, 5629–5645 (2016).
Hübscher, V., Mudholkar, K. & Rospert, S. The yeast Hsp70 homolog Ssb: a chaperone for general de novo protein folding and a nanny for specific intrinsically disordered protein domains. Curr. Genet doi:10.1007/s00294-016-0610-6 (2016).
Gautschi, M. et al. RAC, a stable ribosome-associated complex in yeast formed by the DnaK-DnaJ homologs Ssz1p and zuotin. Proc. Natl Acad. Sci. USA 98, 3762–3767 (2001).
Conz, C. et al. Functional characterization of the atypical Hsp70 subunit of yeast ribosome-associated complex. J. Biol. Chem. 282, 33977–33984 (2007).
Fiaux, J. et al. Structural analysis of the ribosome-associated complex (RAC) reveals an unusual Hsp70/Hsp40 interaction. J. Biol. Chem. 285, 3227–3234 (2010).
Hundley, H. et al. The in vivo function of the ribosome-associated Hsp70, Ssz1, does not require its putative peptide-binding domain. Proc. Natl Acad. Sci. USA 99, 4203–4208 (2002).
Gautschi, M., Mun, A., Ross, S. & Rospert, S. A functional chaperone triad on the yeast ribosome. Proc. Natl Acad. Sci. USA 99, 4209–4214 (2002).
Lopez, N., Halladay, J., Walter, W. & Craig, E. A. SSB, encoding a ribosome-associated chaperone, is coordinately regulated with ribosomal protein genes. J. Bacteriol. 181, 3136–3143 (1999).
Albanese, V. et al. Systems analyses reveal two chaperone networks with distinct functions in eukaryotic cells. Cell 124, 75–88 (2006).
Peisker, K. et al. Ribosome-associated complex binds to ribosomes in close proximity of Rpl31 at the exit of the polypeptide tunnel in yeast. Mol. Biol. Cell 19, 5279–5288 (2008).
Leidig, C. et al. Structural characterization of a eukaryotic chaperone--the ribosome-associated complex. Nat. Struct. Mol. Biol. 20, 23–28 (2013).
Zhang, Y. et al. Structural basis for interaction of a cotranslational chaperone with the eukaryotic ribosome. Nat. Struct. Mol. Biol. 21, 1042–1046 (2014).
Huang, P. et al. The Hsp70 Ssz1 modulates the function of the ribosome-associated J-protein Zuo1. Nat. Struct. Mol. Biol. 12, 497–504 (2005).
Amlacher, S. et al. Insight into structure and assembly of the nuclear pore complex by utilizing the genome of a eukaryotic thermophile. Cell 146, 277–289 (2011).
van Noort, V. et al. Consistent mutational paths predicteukaryotic thermostability. BMC Evol. Biol. 13, 7 (2013).
Dragovic, Z. et al. Fes1p acts as a nucleotide exchange factor for the ribosome-associated molecular chaperone Ssb1p. Biol. Chem. 387, 1593–1600 (2006).
Raviol, H. et al. Chaperone network in the yeast cytosol: Hsp110 is revealed as an Hsp70 nucleotide exchange factor. EMBO J. 25, 2510–2518 (2006).
Willmund, F. et al. The cotranslational function of ribosome-associated Hsp70 in eukaryotic protein homeostasis. Cell 152, 196–209 (2013).
Arnold, K., Bordoli, L., Kopp, J. & Schwede, T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22, 195–201 (2006).
Biasini, M. et al. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 42, W252–W258 (2014).
Schwede, T., Kopp, J., Guex, N. & Peitsch, M. C. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res. 31, 3381–3385 (2003).
Vogel, M., Mayer, M. P. & Bukau, B. Allosteric regulation of Hsp70 chaperones involves a conserved interdomain linker. J. Biol. Chem. 281, 38705–38711 (2006).
Kabsch, W. & Holmes, K. C. The actin fold. FASEB J. 9, 167–174 (1995).
Zhu, X. et al. Structural analysis of substrate binding by the molecular chaperone DnaK. Science 272, 1606–1614 (1996).
Shulga, N., James, P., Craig, E. A. & Goldfarb, D. S. A nuclear export signal prevents Saccharomyces cerevisiae Hsp70 Ssb1p from stimulating nuclear localization signal-directed nuclear transport. J. Biol. Chem. 274, 16501–16507 (1999).
Granneman, S., Kudla, G., Petfalski, E. & Tollervey, D. Identification of protein binding sites on U3 snoRNA and pre-rRNA by UV cross-linking and high-throughput analysis of cDNAs. Proc. Natl Acad. Sci. USA 106, 9613–9618 (2009).
Granneman, S., Petfalski, E., Swiatkowska, A. & Tollervey, D. Cracking pre-40S ribosomal subunit structure by systematic analyses of RNA-protein cross-linking. EMBO J. 29, 2026–2036 (2010).
Granneman, S., Petfalski, E. & Tollervey, D. A cluster of ribosome synthesis factors regulate pre-rRNA folding and 5.8S rRNA maturation by the Rat1 exonuclease. EMBO J. 30, 4006–4019 (2011).
Qi, R. et al. Allosteric opening of the polypeptide-binding site when an Hsp70 binds ATP. Nat. Struct. Mol. Biol. 20, 900–907 (2013).
Jaiswal, H. et al. The chaperone network connected to human ribosome-associated complex. Mol. Cell Biol. 31, 1160–1173 (2011).
Meyer, A. E. et al. The specialized cytosolic J-protein, Jjj1, functions in 60S ribosomal subunit biogenesis. Proc. Natl Acad. Sci. USA 104, 1558–1563 (2007).
Ben-Shem, A. et al. The structure of the eukaryotic ribosome at 3.0A resolution. Science 334, 1524–1529 (2011).
Pech, M., Spreter, T., Beckmann, R. & Beatrix, B. Dual binding mode of the nascent polypeptide-associated complex reveals a novel universal adapter site on the ribosome. J. Biol. Chem. 285, 19679–19687 (2010).
Nyathi, Y. & Pool, M. R. Analysis of the interplay of protein biogenesis factors at the ribosome exit site reveals new role for NAC. J. Cell Biol. 210, 287–301 (2015).
Greber, B. J., Boehringer, D., Montellese, C. & Ban, N. Cryo-EM structures of Arx1 and maturation factors Rei1 and Jjj1 bound to the 60S ribosomal subunit. Nat. Struct. Mol. Biol. 19, 1228–1233 (2012).
Green, M. R. & Sambrook, J. in Molecular Cloning: a Laboratory Manual 4th edn (eds Green, M. R., Sambrook, J.) p. 1-2028 (Inglis, 2012).
Rakwalska, M. & Rospert, S. The ribosome-bound chaperones RAC and Ssb1/2p are required for accurate translation in Saccharomyces cerevisiae. Mol. Cell Biol. 24, 9186–9197 (2004).
Gietz, R. D. & Sugino, A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74, 527–534 (1988).
O'Brien, M. C., Flaherty, K. M. & McKay, D. B. Lysine 71 of the chaperone protein Hsc70 Is essential for ATP hydrolysis. J. Biol. Chem. 271, 15874–15878 (1996).
Janke, C. et al. A versatile toolbox for PCR-based tagging of yeast genes: new fluorescent proteins, more markers and promoter substitution cassettes. Yeast 21, 947–962 (2004).
Longtine, M. S. et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961 (1998).
Kabsch, W. XDS. Acta Crystallogr. Sect. D Biol. Crystallogr 66, 125–132 (2010).
Bunkoczi, G. et al. Phaser.MRage: automated molecular replacement. Acta Crystallogr. Sect. D Biol. Crystallogr. 69, 2276–2286 (2013).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. Sect. D Biol. Crystallogr. 66, 213–221 (2010).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. Sect. D Biol. Crystallogr. 66, 486–501 (2010).
Afonine, P. V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. Sect. D Biol. Crystallogr. 68, 352–367 (2012).
Murshudov, G. N. et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. Sect. D Biol. Crystallogr. 67, 355–367 (2011).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 66, 12–21 (2010).
Schrödinger, L. L. C. The PyMOL Molecular Graphics System, Version 1.8 (2015).
Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Baker, N. A. et al. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl Acad. Sci. USA 98, 10037–10041 (2001).
Dolinsky, T. J. et al. PDB2PQR: expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucleic Acids Res. 35, W522–W525 (2007).
Dolinsky, T. J., Nielsen, J. E., McCammon, J. A. & Baker, N. A. PDB2PQR: an automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res. 32, W665–W667 (2004).
Sievers, F. et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (2011).
Robert, X. & Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 42, W320–W324 (2014).
Ashkenazy, H. et al. ConSurf 2010: calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res. 38, W529–W533 (2010).
Celniker, G. et al. ConSurf: using evolutionary data to raise testable hypotheses about protein function. Isr. J. Chem. 53, 199–206 (2013).
Thoms, M. et al. The exosome is recruited to RNA substrates through specific adaptor proteins. Cell 162, 1029–1038 (2015).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Stevens, S. W. & Abelson, J. Yeast pre-mRNA splicing: methods, mechanisms, and machinery. Methods Enzymol. 351, 200–220 (2002).
Ashe, M. P., De Long, S. K. & Sachs, A. B. Glucose depletion rapidly inhibits translation initiation in yeast. Mol. Biol. Cell 11, 833–848 (2000).
Zhang, Y., Wölfle, T. & Rospert, S. Interaction of nascent chains with the ribosomal tunnel proteins Rpl4, Rpl17, and Rpl39 of Saccharomyces cerevisiae. J. Biol. Chem. 288, 33697–33707 (2013).
Zhang, Y. et al. NAC functions as a modulator of SRP during the early steps of protein targeting to the endoplasmic reticulum. Mol. Biol. Cell 23, 3027–3040 (2012).
Zhang, Y. et al. Cotranslational intersection between the SRP and GET targeting pathways to the ER of Saccharomyces cerevisiae. Mol. Cell Biol. 36, 2374–2383 (2016).
Landau, M. et al. ConSurf 2005: the projection of evolutionary conservation scores of residues on protein structures. Nucleic Acids Res. 33, W299–W302 (2005).
Cannone, J. J. et al. The comparative RNA web (CRW) site: an online database of comparative sequence and structure information for ribosomal, intron, and other RNAs. BMC Bioinf 3, 2 (2002).
Acknowledgements
We thank J. Kopp, C. Siegmann and G. Müller from the BZH/Cluster of Excellence:CellNetworks crystallization platform for protein crystallization, and acknowledge access to the beamlines at the European Synchrotron Radiation Facility (ESRF) in Grenoble and the support of the beamline scientists. We thank K. Bendrin for help with the CRAC methodology and D. Ibberson at the CellNetworks Deep Sequencing Core Facility for performing HiSeq sequencing. We thank E. Hurt, S. Amlacher and M. Thoms for providing Saccharomyces cerevisiae strains and plasmids used for CRAC analysis and Chaetomium thermophilum cDNA. We acknowledge D. Layer and A. Hendricks for technical assistance, K. Wild and M. Mayer for helpful discussions, R. Kityk and M. Mayer for sharing experimental protocols and access to the ultraviolet-spectropolarimeter.
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) through FOR967 (to I.S. and S.R.), GRK1188 and the Leibniz programme (to I.S.), SFB 746 (to S.R.), and RO 1028/5-1 (to S.R.), and by HBIGS fellowships (to G.V.G. and F.A.W.), and by the Excellence Initiative of the German federal and state governments (BIOSS-2) (to S.R). I.S. is an investigator of the Cluster of Excellence: CellNetworks.
Author information
Authors and Affiliations
Contributions
A.G., C.C., G.V.G., Y.Z., F.A.W., K.L., U.v.P., S.R. and I.S. designed the experiments, analysed the data and wrote the manuscript. A.G., C.C., G.V.G., Y.Z., F.A.W., K.L., J.K., U.v.P., E.F. and T.W. performed experiments, G.S. and T.F. processed and discussed data. All authors read and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1 - 32, Supplementary Tables 1 - 3, Supplementary Methods and Supplementary References (PDF 121278 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Gumiero, A., Conz, C., Gesé, G. et al. Interaction of the cotranslational Hsp70 Ssb with ribosomal proteins and rRNA depends on its lid domain. Nat Commun 7, 13563 (2016). https://doi.org/10.1038/ncomms13563
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms13563
This article is cited by
-
High-resolution structures of a thermophilic eukaryotic 80S ribosome reveal atomistic details of translocation
Nature Communications (2022)
-
Ribosome-bound Get4/5 facilitates the capture of tail-anchored proteins by Sgt2 in yeast
Nature Communications (2021)
-
Pathway of Hsp70 interactions at the ribosome
Nature Communications (2021)
-
Conformational dynamics of free and membrane-bound human Hsp70 in model cytosolic and endo-lysosomal environments
Communications Biology (2021)
-
The ribosome-associated complex RAC serves in a relay that directs nascent chains to Ssb
Nature Communications (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.