Abstract
Expansion of the cytochrome P450 gene family is often proposed to have a critical role in the evolution of metabolic complexity, in particular in microorganisms, insects and plants. However, the molecular mechanisms underlying the evolution of this complexity are poorly understood. Here we describe the evolutionary history of a plant P450 retrogene, which emerged and underwent fixation in the common ancestor of Brassicales, before undergoing tandem duplication in the ancestor of Brassicaceae. Duplication leads first to gain of dual functions in one of the copies. Both sister genes are retained through subsequent speciation but eventually return to a single copy in two of three diverging lineages. In the lineage in which both copies are maintained, the ancestral functions are split between paralogs and a novel function arises in the copy under relaxed selection. Our work illustrates how retrotransposition and gene duplication can favour the emergence of novel metabolic functions.
Similar content being viewed by others
Introduction
Evolution of new protein functions is often initiated by gene duplication, but most duplicates are discarded by purifying selection1,2,3,4,5. The retention of new copies may be explained by either neofunctionalization or subfunctionalization1,6,7, with several models for subfunctionalization proposed5,7,8,9,10,11. Functional innovation may result from an expansion of a previous biochemical activity or from a change in the biochemical properties of the protein12,13. Acquisition of an additional biological activity can then lead to a pleiotropic protein. Multiple analyses have shown that the evolutionary scenario after gene duplication may be more complex, implicating several of these mechanisms, acting one after another14,15,16, as has been suggested by theory17. However, experimental evidence of the models and their interplay is often difficult to obtain. We describe here the evolutionary history of a plant retrogene18 that stemmed at an early step of Brassicales evolution.
The parent gene, represented by CYP98A3 in Arabidopsis thaliana (family Brassicaceae, order Brassicales), controls a major branch point in the plant phenolic pathway19,20. It is broadly expressed in plants, especially in vascular tissues, and encodes the phenolic ring meta-hydroxylase of lignin precursors. This enzyme evolved in land plants around 400 million years ago (Ma) to provide metabolites essential for protection against desiccation and ultraviolet radiation, water conduction and erect growth21. Due to its gate-keeping role and the high flux in the lignin pathway, CYP98A3 and its orthologs are under strong purifying selection18. Only a few lineage-specific duplications are inferred in phylogenies of CYP98A3 across land plants. One such duplication occurred via transposition within Brassicales and was followed by a tandem duplication giving rise to CYP98A8 and CYP98A9 genes leading to the formation of N1,N5-di(hydroxyferuloyl)-N10-sinapoyl-spermidine, a major pollen coat and wall constituent in A. thaliana18,22. Available data indicate that both CYP98A8 and CYP98A9 catalysed the first meta-hydroxylation of the phenolic ring of pollen constituents (3′-hydroxylation of N1,N5,N10-tri-coumaroyl-spermidine) and that CYP98A8 also catalysed the second (5′-hydroxylation of N1,N5,N10-tri-feruloyl-spermidine). The recent emergence of this pathway and the sequencing of a representative set of Brassicales genomes offers the opportunity to determine the evolutionary process leading to these new P450 functions.
We show here that the founding retroposition leading to the CYP98A8 and CYP98A9 common ancestor occurred before the divergence of Cleomaceae and was followed by a duplication before the divergence of the core Brassicaceae lineages. We demonstrate that the fate and function of the resulting paralogs differ between the Brassicaceae lineages, with either loss of one of the paralogs, the other maintaining a dual function in lineage II and III, or conservation of the two gene copies in lineage I, with subfunctionalization and gain of an additional activity in flavonoid metabolism by one of the duplicates.
Results
Evolutionary history of the CYP98 family in Brassicales
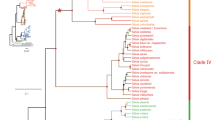
A large set of genomes in Brassicales has now been sequenced, allowing the reconstruction of CYP98A8 and CYP98A9 evolutionary history (Fig. 1a; Supplementary Fig. 1 and Supplementary Table 1). The reconstructed CYP98 phylogeny first shows that a CYP98A8/9 homolog is found in some, but not all Brassicales. For example, Tarenaya hassleriana, belonging to Cleomaceae, the sister family that split from Brassicaceae around 70 Ma23,24, contains a CYP98A8/9 copy, but the more early diverging Carica papaya (Caricaceae; 100 MY divergence with Brassicaceae) does not. This dates the CYP98A8/9 founding retroposition between 70 and 100 Ma25. The tandem duplication generating the CYP98A8 and CYP98A9 paralogs occurred after the divergence of the genus Aethionema, predating the divergence of the major lineages I, II and III of core Brassicaceae (Fig. 1a; ref. 23). The duplication pattern indicates that lineages II and III subsequently lost CYP98A9, while lineage I retained it. A CYP98A8 ortholog is present in all available Brassicaceae genomes, which include lineage I and lineage II species23, but CYP98A9 is found only in lineage I (Fig. 1a).
(a) Annotated protein maximum likelihood tree illustrating the phylogenetic relationships in the CYP98 family in Brassicales. The CYP98A3 clade is the parent to CYP98A8/9s. Evolution regimes (ω=dN/dS) computed with codeml Branch model and acting on different phylogenetic groups are indicated. Branch support in the tree was calculated from 1,000 bootstraps replicates. (b) In vitro assay of CYP98A8/A9 enzymes activities. Tri-coumaroyl-spermidine and tri-feruloyl-spermidine were used to assess the 3′- and 5′-hydroxylase activities of yeast-expressed proteins. CYP98A8/A9 from all species have 3′-hydroxylase activity. Only CYP98A8 from Brassicaceae have 5′-hydroxylase activity. (c) Search for sinapoylated (m/z 207) phenolamides by parent ion scan analysis of flower buds demonstrates the absence in vivo of the tri-hydroxylated phenolamide di-(hydroxyferuloyl)-sinapoyl-spermidine (m/z 736) in A. grandiflorum, while it is present in Brassicaceae from lineages I, II and III. Colour of each profile is according to the lineage colour code in a.
In the absence of sequenced lineage III species, we designed degenerate primers to amplify CYP98A8/9 homologs from the lineage III species Chorispora tenella and Euclidium syriacum. A CYP98A8 orthologous sequence could be retrieved from both species (Supplementary Fig. 2), but no CYP98A9. However, BLAST search of the CYP98A8 flanking regions, respectively, found one and four correctly ordered remnant CYP98A9 fragments in C. tenella and E. syriacum, respectively (Supplementary Fig. 2), indicating that CYP98A9 had been present and was subsequently lost from the genome. The presence of a fragmented CYP98A9 confirms the duplication history inferred from the gene tree. Thus, the tandem duplication event that gave birth to CYP98A9 predates the split between sister lineages I, II and III, around 45 Ma, and CYP98A9 is retained only in lineage I.
Activity of the CYP98A8/9 proteins in vivo and in vitro
We then assessed CYP98 enzyme activity in each lineage using two complementary approaches. One was the yeast-expression of representative CYP98A8/A9 genes for in vitro assay of catalytic activity (Fig. 1b). The second employed metabolic profiling of flower buds from members of each Brassicaceae lineage and a representative of the earliest diverging branch in the family (that is, the genus Aethionema) (Fig. 1c). In vitro assays revealed that: (1) the CYP98A8/9 protein from Adenium arabicum and T. hassleriana catalysed only 3′-hydroxylation of tri-coumaroyl-spermidine; (2) the radiation of core Brassicaceae was preceded by acquisition of the 5′-hydroxylase activity, shown by the ability of CYP98A8 from Eutrema salsugineum (lineage II) and A. thaliana (lineage I) to catalyse both 3′-hydroxylation of tri-coumaroyl-spermidine and 5′-hydroxylation of tri-feruloyl-spermidine; (3) A. thaliana CYP98A9 has 3′- but not 5′-hydroxylase activity, similar to A. arabicum and T. hassleriana. Taken together, our in vitro data suggest that 5′-hydroxylase activity evolved in CYP98A8, post duplication.
Phenolic profiling supported the results from the enzyme assays. The 5′-hydroxylated phenolamide di-(hydroxyferuloyl)-sinapoyl-spermidine was absent from the flowers of Aethionema grandiflorum, but present as at least two different isomers in core Brassicaceae (Fig. 1c; Supplementary Fig. 3). Hence, we inferred that the single CYP98A8/9 copy in the ancestor of both Brassicaceae and Cleomaceae lacked 5′-hydroxylase activity. Moreover, the single CYP98A8 copy present in lineages II and III performed both 3′- and 5′-hydroxylations, in vitro and in vivo. This finding is also supported for lineage I species by the ability of A. thaliana CYP98A8 to catalyse both reactions in vitro. Both sets of analyses thus concurred to indicate that the acquisition of 5′-hydroxylation occurred in the ancestor of core Brassicaceae. However, whether this acquisition predated, or was subsequent to, the gene duplication that gave rise to CYP98A9 remained an open question.
To address this question we took advantage of a recently released A. thaliana cyp98a9 insertion mutant (Supplementary Fig. 4) and compared its young flower bud phenolic profile to those of the previously characterized cyp98a8 insertion line and wild-type plants. Ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) profiles of the flower buds revealed a dramatic decrease in the major phenolamide compound N1,N5-di-(hydroxyferuloyl)-N10-sinapoyl-spermidine in both the cyp98a8 and cyp98a9 single mutants, with a concomitant accumulation of N1,N5,N10-tri-feruloyl-spermidine in cyp98a8. The latter compound was completely absent in cyp98a9 buds (Fig. 2). This confirmed that subfunctionalization of CYP98A8 and CYP98A9 in the phenolamide pathway occurred in lineage I. The complete partitioning of functions in vivo, when 3′-hydroxylase activity is shared by both enzymes tested in vitro, implied a modification in protein–protein interaction resulting in spatial reorganization of partner enzymes in the tapetal cells. We then hypothesized that the split of function we detected in lineage I most likely contributed to the retention of CYP98A9.
(a) The anther-specific phenolamide pathway as previously delineated in A. thaliana. (b–e) Targeted metabolic profiling in A. thaliana flower buds shows that phenolamide intermediates downstream of N1,N5,N10-tri-coumaroyl-spermidine are absent from the cyp98a9 mutant, indicating that CYP98A8 does not perform the 3′-hydroxylation in vivo: (b) N1,N5,N10-tri-coumaroyl-spermidine, (c) N1,N5,N10-tri-feruloyl-spermidine, (d) N1,N5,N10-tri-(hydroxyferuloyl)-spermidine, (e) N1,N5-di-(hydroxyferuloyl)-N10-sinapoyl-spermidine. Results are the mean±s.e. of three independent determinations. Reversible activity of the spermidine:hydroxycinnamoyl transferase acting upstream of CYP98A9 presumably accounts for the lower accumulation of the N1,N5,N10-tri-coumaroyl-spermidine in the cyp98a9 mutant compared with the accumulation of N1,N5,N10-tri-feruloyl-spermidine in cyp98a8.
Molecular evolution of the CYP98 duplicates in Brassicales
To gain some insight into the molecular evolutionary mechanism that led to functional shifts in CYP98A8 and CYP98A9, we compared rates of non-synonymous to synonymous codon substitutions (omega, ω=dN/dS) along several branches of the Brassicales CYP98 phylogeny. Both the branch model and the clade model of Codeml analyses indicated that all CYP98 sequences were under purifying selection (all ω<0.4, and even 0.2 for the branch model) and that the lineage I CYP98A8 sequences were significantly more constrained than those of CYP98A9 and CYP98A8 from lineages II and II (Fig. 1a; Supplementary Note 1; Supplementary Table 2). Interestingly, when applying the free-ratio model of Codeml (independent ω ratio for each branch), we detected a relaxation of selection occurring along the branch leading to the CYP98A8 clade (ω=0.63; Supplementary Fig. 5), which could reflect the acquisition of the 5′-hydroxylase activity in CYP98A8. With a single exception, ω values were below 1; ω for lineage I CYP98A3 and CYP98A8 sequences was lower than for CYP98A8s from lineages II and III. The highest ω was associated to the basal internal branch of the CYP98A9 clade that showed evidence of relaxed selection (ω=1.01; Supplementary Fig. 5), which suggested the possibility of a change of function emerging post-duplication in CYP98A9s. A McDonald–Kreitman test comparing within-population polymorphism with between-species divergence26 for A. thaliana and L. alabamica CYP98A9s showed a significant signature of purifying selection acting on A. thaliana CYP98A9 proteins (Supplementary Table 3). All models, including Codeml branch-site and Fitmodel analysis (Supplementary Note 1; Supplementary Table 2), failed to detect a significant signature of positive selection along any branch of the tree and/or sequence sites.
CYP98A8 and CYP98A9 wire to different regulatory networks
To examine the possibility of a change of function emerging in CYP98A9 after duplication, we first analysed in more detail the CYP98A9 expression pattern in A. thaliana. CYP98A9 was previously shown to share CYP98A8 expression during pollen development, but its promoter was also active in root tips and revealed weak expression in vascular tissues18. Functional divergence following the tandem duplication is further suggested by a co-expression analysis indicating a strong correlation of CYP98A8 expression with a major network of genes involved in flower development, whereas CYP98A9 expands to an additional network related to seeds (Fig. 3a–c; Supplementary Table 4). This observation was confirmed by β-glucuronidase (GUS) staining, which showed CYP98A9 promoter activity in mature, green seed endosperm (Fig. 3d), consistent with a recent microarray analysis27. In sum, both our own and published expression data indicate that the role of CYP98A9 reached beyond pollen metabolism.
(a) AtCYP98A8 and (b) AtCYP98A9 co-expression networks. AtCYP98A9 is associated with two distinct co-expression clusters, labelled A and B. (c) Hierarchical clustering of genes involved in the different co-expression networks. Each element of the matrix represents the expression potential of a given gene (column) in a defined anatomic compartment (line). Blue arrowheads indicate flower- and male reproductive tissue-related samples. Red arrowheads indicate seed-related samples. (d) Analysis of pCYP98A8:uidA and pCYP98A9:uidA expression patterns in opening flowers and green seeds.
CYP98A9 gained an additional activity in flavonoid metabolism
To reveal potential additional functions and overlooked activities of CYP98A9, we used CYP98A9 loss and gain of function lines. The latter were generated using the promoter of the upstream P450 gene in the phenolic pathway, encoding the cinnamic acid 4-hydroxylase (C4H)28 that provides precursors for the lignin, phenolamide and flavonoid pathways. A similar CYP98A8 overexpression construct was used as control. We observed no differences in development, seed set or colour, and fertility between wild-type and cyp98a9 null mutants grown under controlled conditions. No obvious alteration in plant growth, seed set and phenotype was likewise detected for pC4H:AtCYP98A8. Conversely, seeds from pC4H:AtCYP98A9-transformed plants showed a typical transparent testa (tt) phenotype (yellow instead of brown seeds) (Fig. 4a), typical of seed coats deficient in flavonoids and tannins29,30. Vanillin seed staining of epicatechins and proanthocyanidins31 confirmed that only CYP98A9 expression led to a seed coat tannins defect (Supplementary Fig. 6a). Moreover, UPLC-MS/MS profiling of soluble flavonoids indicated that AtCYP98A9 expression resulted in a complete depletion in quercetin-3-O-rhamnoside (Supplementary Table 5), the major soluble seed flavonol in A. thaliana32. CYP98A9 expression thus impacts soluble flavonoid metabolism and not just condensed tannin composition or polymerization. However, no significant alteration in whole seed, flower buds or developing siliques flavonoids was detected in the insertion mutant cyp98a9 (Fig. 4a; Supplementary Figs 6b and 7), but time- or cell-specific alteration in flavonoid content in this mutant cannot be excluded.
(a) Colour of the seeds from A. thaliana transgenic lines expressing CYP98A8/A9 genes under control of the A. thaliana C4H promoter. Only expression of lineage I CYP98A9 leads to a transparent testa (tt) phenotype. Col-4: wild-type Arabidopsis Columbia-4. Ler: wild-type Arabidopsis Landsberg erecta. Ws: wild-type Arabidopsis Wassilewskija. tt4-1: transparent testa 4 mutant for the chalcone synthase gene. (b) In vitro enzyme assays showing the CYP98A9-dependent conversion of naringenin into eriodictyol. Assays with (+) and controls without (−) NADPH were performed. Eriodictyol (grayed elution time) was detected using the m/z 289.4>153.0 transition in multiple reactions monitoring (MRM) positive mode. (c) Relative naringenin hydroxylase activity (% relative to highest activity set to 100). Error bars represent the s.e. from three independent assays. Aa, Aethionema arabicum; At, Arabidospsis thaliana; Es, Eutrema salsugineum.
To determine if this flavonoid-impacting function was specific to A. thaliana CYP98A9, overexpression constructs of another lineage I CYP98A9 (CrCYP98A9, Capsella rubella), and of representative members of lineage II CYP98A8 (EsCYP98A8, E. salsugineum) and of the ancestral AaCYP98A8/9 (A. arabicum) were tested for their impact on seeds. Only expression of C. rubella CYP98A9 prevented seed flavonoid accumulation (Fig. 4a; Supplementary Fig. 6b). The capacity to impair flavonoid accumulation was thus likely acquired by CYP98A9 before the radiation of lineage I. We then set out to identify the flavonoid substrate of CYP98A9s likely to explain the transparent testa phenotype. In vitro enzymatic screens of flavonoid candidates spotted the AtCYP98A9-dependent conversion of naringenin into eriodictyol (that is, 3′-hydroxy-naringenin), a reaction very poorly catalysed by AtCYP98A8, EsCYP98A8 and AaCYP98A8/9 (Fig. 4b,c). AtCYP98A8 and AtCYP98A9 homology modelling and docking experiments in addition confirmed that only AtCYP98A9 efficiently anchored S-naringenin in its active site, with B-ring oriented for 3′-hydroxylation (Fig. 5a; Supplementary Fig. 8). From the three residues critical for naringenin stabilization, only Lys209 was found discriminant throughout the CYP98A8s/CYP98A9s alignment (Supplementary Fig. 9). The involvement of Lys209 in AtCYP98A9 naringenin hydroxylase activity was supported by Lys209 substitution with uncharged amino acids (Ala and Ile) leading to up to 50% selective decrease in flavonoid hydroxylase activity (Fig. 5b). According to Fitmodel results (MX+S1 model, Fig. 5c; Supplementary Note 1; Supplementary Table 2) used to estimate different dN/dS ratios to each sequence site and branch on the phylogenetic tree (see Methods), this site was constrained in CYP98A9 sequences (ω=0.038—codon AAG in the six species analysed; Supplementary Fig. 9), but evolved neutrally (ω=1.000) at the basal branch of the A9 clade and in most of the other branches of the A8A9 clade (Fig. 5c). Lys209 and the surrounding residues that also vary between CYP98A8 and CYP98A9 might thus be instrumental in the further evolution of CYP98A9 function.
(a) Docking of S-naringenin in the AtCYP98A9 active site. Arg96, Trp109 and Lys209 anchor naringenin for B-ring 3′-hydroxylation. (b) Lys209 substitution with alanine or isoleucine reduces AtCYP98A9 naringenin hydroxylase activity in vitro. Error bars represent the s.e. from three independent assays. (c) Fitmodel reconstitution of the history of the selection pressure on the AtCYP98A9 Lys209 codon. The most frequent codon and amino acid are indicated on branches.
It is possible that the novel CYP98A9 function is still latent, yet flavonoids are regarded as important determinants of plant fitness as ultraviolet protectors, antimicrobials and antifeedants33. They are reported to impact seed germination and growth34. It thus can also be postulated that the new function of CYP98A9 contributed to its retention in lineage I.
Discussion
In summary, our work provides an unprecedented example of multistep and divergent evolution of a retrogene expressed in male reproductive tissues, where successive duplication, along with acquisition and partitioning of a dual function, led to progressive, lineage-specific expansion of phenolic metabolism, and emergence of new and diverse metabolic capacities in the plant. Our experimental data are fully supported by molecular evolutionary results and highlight the rise of an extended gene activity simultaneously rewiring gene expression networks and leading to an enzyme with pleiotropic activity. Metabolic profiling of plant tissues and ectopic gene expression provide a practical demonstration that in vitro enzyme activity and gene expression in a single context are not sufficient to describe gene function. Our data, in addition, reveal a striking case of divergent evolution of paralogs in sister lineages (Fig. 6), illustrating how relaxation of purifying selection following gene duplication can allow for the exploration of novel metabolic pathways.
(a) Initial steps of the CYP98A8/9 evolution shared by lineages I, II and III. The initial CYP98A3 retroposition event in Brassicales (100-70 Ma) led to the CYP98A8/9 ancestral gene, which became a phenolamide 3′-hydroxylase. The retroposition was followed by a tandem gene duplication in Brassicaceae around 45 Ma leading to the CYP98A8 and CYP98A9 sister genes. CYP98A8 then acquired an additional function, the phenolamide 5′-hydrolase activity. (b) Further evolution of CYP98A8 and CYP98A9 in the lineage I of the Brassicaceae, CYP98A8 lost the phenolamide 3′-hydroxylase activity in vivo, and maintained only the 5′-hydroxylase activity. Meanwhile, CYP98A9 maintained the 3′-hydroxylase activity, and simultaneously gained naringenin 3′-hydroxylase activity. (c) Further evolution of CYP98A8 and CYP98A9 in the lineages II and III of the Brassicaceae, in lineages II and III, the CYP98A9 copy was lost, and CYP98A8 maintained both 3′- and 5′-hydroxylase activities.
Methods
Plant materials and growth conditions
Unless otherwise stated, plants were grown on soil under a 16h/8h (day/night) regime with 70% humidity. The cyp98a8 insertion mutant (SALK_131366) was isolated and characterized previously18. The cyp98a9 insertion mutant (#SK27279) was obtained from the Saskatoon transfer DNA collection35. The correct transfer DNA insertion in the CYP98A9 gene was confirmed by PCR-based genotyping and further validated by the drastic decrease in CYP98A9 expression observed in the mutant by quantitative reverse transcription PCR (qRT–PCR) (Supplementary Fig. 4). Primers used for the mutant validation are listed in Supplementary Table 6. For parent ion scan analysis, flower buds from A. thaliana and E. salsugineum grown under controlled conditions were harvested. Flower buds from T. hassleriana were a kind gift from Pr. Eric Schranz (Wageningen University). Bunias orientalis and A. grandiflorum were sampled in the botanical garden of the University of Strasbourg.
Cloning procedures and production of transgenic lines
The intronless coding sequences of AtCYP98A8, AtCYP98A9, CrCYP98A9, EsCYP98A8 and AaCYP98A8/9 were PCR-amplified from genomic DNA using Gateway compatible primers and introduced into pDONR207 vector by BP reaction to generate pENTRY clones. Expression constructs pC4H:AtCYP98A8, pC4H:AtCYP98A9, pC4H:CrCYP98A9, pC4H:EsCYP98A8 and pC4H:AaCYP98A8/9 were obtained by recombining the corresponding pENTRY with the pCC0996 Gateway destination vector that contains a 3 kb promoter fragment of A. thaliana Cinnamate 4-Hydroxylase (pC4H)36 using LR clonase (Invitrogen). Primers used for molecular cloning are listed in Supplementary Table 6. Agrobacterium tumefaciens GV3101 carrying the expression vector was used to transform Arabidopsis wild-type plants (Ws background) by dipping immature inflorescences in a bacterial suspension37. Transgenic plants were selected on soil via Basta treatment (250 mg l−1; Agro Evo). T2 seeds were used for subsequent analyses.
Amplification of CYP98A region from lineage III species
To obtain CYP98A8 and CYP98A9 sequence data from species in Brassicaceae lineage III, we performed multiple rounds of high-efficiency thermal asymmetric interlaced PCR (hiTAIL-PCR)38. Initial nested primer sets were designed to anneal to regions highly conserved in known Brassicaceae CYP98A8 and CYP98A9 sequences. Polymorphic sites were incorporated into primers, and thus the resulting primers were degenerate at several nucleotide positions. Amplicons were sequenced and used to inform sequence-specific primer design to walk upstream and downstream along the chromosome using additional rounds of hiTAIL-pPCR38. Primers are available in Supplementary Table 6.
E. syriacum and C. tenella DNA samples were those used in Beilstein et al.39. Nested amplification was performed in a two-step process according to the hiTAIL-PCR protocol38. The process was repeated until the assembled reads reached flanking genes. The software CoGe40 was used to compare assembled sequence reads for E. syriacum and C. tenella with published whole-genome sequences from Brassicaceae lineage I and II species.
PCR products were separated on a 1% agarose gel with low EDTA before excision, filtration (Millapore Centrifugal Filter Units), and ethanol precipitation. Purified PCR products were ligated into pGEM-T Easy vector (Promega) and transformed into TOP10 competent cells and plated on selective media for screening. Positive transformants were cultured overnight in liquid media, and plasmid DNA harvested using an alkaline lysis kit (Zymo Research). Cloned fragments were sequenced in both directions to achieve a minimum of 85% overlap. Overlapping sequence reads were assembled into a consensus sequence using the software Geneious v. 6.6 (Biomatters).
Recombinant CYP98 protein production in yeast
Intronless coding sequences of EsCYP98A8, AaCYP98A8/9 and ThCYP98A8/9 genes were PCR-amplified from genomic DNA using USER-compatible primers41. Amplicons were subsequently introduced in the pYeDP60u2 vector42 and verified by sequencing. For AtCYP98A8 and AtCYP98A9 pYeDP60 plasmids construction, the corresponding coding sequences were PCR-amplified from genomic DNA and introduced between the BamHI and KpnI sites and BamHI and EcoRI sites, respectively, as described previously18. Primers used for molecular cloning are visible in Supplementary Table 6.
The Saccharomyces cerevisiae WAT11 strain, expressing the ATR1 cytochrome P450 reductase from A. thaliana43, was transformed with pYeDP60 vectors and selected on minimum SGI medium (1 g l−1 bactocasamino acids, 7 g l−1 yeast nitrogen base, 20 g l−1 glucose and 40 mg l−1 L-tryptophan). Liquid cultures were initiated from selected colonies grown on SGI for 18 h at 28 °C under agitation (160 r.p.m.). Ten milliliters of SGI culture were used to inoculate 200 ml of YPGE (10 g l−1 bactopeptone, 10 g l−1 yeast extract, 5 g l−1 glucose, 3% ethanol by volume). After 30 h growth at 28 °C at 160 r.p.m., recombinant proteins production was induced by supplementing the medium with 10 ml of 200 g l−1 galactose and further incubation at 20 °C for 16 h.
Yeast cells were harvested by centrifugation at 7,500g for 10 min at 4 °C, washed with TEK buffer (50 mM Tris-HCl pH 7.5, 1 mM EDTA, 100 mM KCl) and resuspended in 2 ml of TES (50 mM Tris-HCl pH 7.5, 1 mM EDTA, 600 mM sorbitol) supplemented with 5 mM 2-mercaptoethanol and 10 g l−1 bovine serum albumin, fraction V (buffer A). Cell suspensions were transferred to 50 ml conical tubes and homogenized with 0.5 mm glass beads by handshaking five times for 1 min each. Beads were washed twice with 30 ml of cold buffer A. Cell debris and remaining glass beads were removed from the pooled lysates by 20 min centrifugation at 7,500g and 4 °C. Supernatant was filtrated on Miracloth (22–25 μm pore size, Calbiochem, Maryland) and microsomal fraction pelleted by centrifugation at 100,000g and 4 °C for 1 h. Pelleted membranes were resuspended in TEG buffer (50 mM Tris-HCl pH 7.5, 0.5 mM EDTA, 30% glycerol by volume) with a Potter-Elvehjem homogenizer. Microsomal membranes were stored at −20 °C until processing.
Enzyme assays
Enzyme assays were conducted in 100 μl final volume, containing 20 mM potassium phosphate buffer (pH 7.4), 200 μg microsomal proteins, 100 μM substrate and 500 μM NADPH. Reactions were initiated with NADPH addition, incubated for 30 min at 28 °C in the dark and terminated with 10 μl of 50% acetic acid. Samples were centrifuged at 13,000g for 5 min to pellet microsomal membranes and supernatants were used for product analysis. Hydroxycinnamoyl-spermidines used as substrates and chromatographic references were chemically synthesized18. For naringenin 3′-hydroxylation, activity was normalized to the activity recorded for the same enzyme with tri-p-coumaroyl-spermidine and expressed as a percentage of the maximal activity.
Extraction and analysis of phenolamides and flavonoids
For flavonoid analysis, mature dry seeds were ground in a 2 ml safe-locker tube with 1 ml of 75% acetonitrile for 5 min using a TissueLyser II (Qiagen) at 4 °C. The extracts were sonicated for 20 min on ice. After centrifugation (14,000g, 15 min), the pellet was further extracted with 1 ml of 75% acetonitrile by overnight shaking at 4 °C. The two extracts were pooled, dried under vacuum and resuspended in 200 μl of 75% acetonitrile before UPLC-MS/MS analysis44.
To release flavonoid aglycones, crude extracts were dried in vacuo. Dry residues were re-suspended in a 50% methanol solution containing 2 N HCl and 2.5 mg ml−1 ascorbic acid as an antioxidant. Acid hydrolysis was performed at 80 °C for 2 h. Samples were cooled down before UPLC-MS/MS analysis.
For the analysis of phenolamides, samples were first extracted with 1 ml of ice-cold 80% methanol. After centrifugation, the supernatant was recovered and the pellet further extracted with 1 ml of ice-cold 80% methanol overnight at 4 °C. Extracts were pooled, dried under vacuum and resuspended in 200 μl of 80% methanol before UPLC-MS/MS analysis. The mobile phase consisted of water (A) and acetonitrile (B), both containing 0.1% formic acid. Run started with 1 min of 95% A, followed by linear gradients to reach 53% B at 7 min and 100% B at 8 min, then isocratic conditions using B for 2.5 min. Return to initial conditions was achieved in 3.5 min, with a total run time of 15 min. The column was maintained at 28 °C with a flow rate of 0.4 ml min−1, injecting 3 or 5 μl sample. Ultraviolet absorbance was recorded from 200 to 600 nm. MS scans in positive and negative mode were performed, and multiple reactions monitoring were developed for every detected peak. Compounds were identified by comparison of two orthogonal data (RT and MS/MS) relative to authentic compounds analysed under identical experimental conditions, or putatively annotated by comparison of MS/MS spectra with published data45,46.
Vanillin assay
Mature dry seeds were incubated in a solution of 1% (w/v) vanillin (Fluka #350346) prepared in 6 N HCl at room temperature for 1 h. Vanillin turns red upon binding to flavan-3,4-diols (leucoanthocyanidins) and flavan-4-ols (catechins), which are present either as monomers or as terminal subunits of proanthocyanidins31.
Determination of gene expression divergence
pCYP98A8:uidA and pCYP98A9:uidA plants18 were incubated in 90% acetone for 20 min on ice, rinsed with water, and transferred to a GUS solution containing 1 mM 5-bromo-4-chloro-3-indolyl-β-D-glucuronide (X-Gluc), 100 mM sodium phosphate (pH 7.0), 10 mM EDTA, 0.5 mM potassium ferricyanide, 0.5 mM potassium ferrocyanide and 0.1% (v/v) Triton X-100. Samples were incubated at 37 °C in the dark overnight. Tissues were cleared three times in 75% ethanol before imaging with a Nikon (ECLIPSE, E800) microscope. For GUS staining of seeds, additional clearing steps were added. Opened siliques were first treated with ethanol:acetic acid (v/v) for 4 h, and then restored in Hoyer’s medium before microsopy analysis47.
Gene co-expression network analysis was performed with the PlaNet on line tool (http://aranet.mpimp-golm.mpg.de/) using At1g74540 (CYP98A8) and At1g74550 (CYP98A9) as gene id inputs. The gene list corresponding to the different co-expression networks (see Supplementary Table 4) were then subjected to hierarchical clustering based on Pearson correlation coefficient and with optimal leaf-ordering using the Genevestigator embedded tool48.
RNA extraction and gene expression analysis by qRT–PCR
Samples were harvested and snap-frozen in liquid nitrogen. Total RNA was isolated from pooled tissues using NucleoSpin RNA Plant kit (Macherey-Nagel) and subsequently treated with DNase I (Fermentas). In all, 1.5 μg of total RNA was reverse-transcribed with SuperScript III Reverse Transcriptase (Invitrogen) in the presence of 200 ng random primers. qRT–PCR plates were prepared with a Biomek 3,000 pipetting system (Beckman Coulter, Villepinte, France) and run on a LightCycler 480 II device (Roche). Each reaction was prepared using 2 μl of tenfold diluted complementary DNA, 5 μl of LightCycler 480 SYBR Green I Master (Roche) and 250 nM of forward and reverse primers in a total volume of 10 μl. The amplification program was 95 °C for 10 min and 40 cycles (95 °C denaturation for 10 s, annealing at 60 °C for 15 s, extension at 72 °C for 15 s), followed by a melting curve analysis from 55 to 95 °C to check for transcripts specificity. All reactions were performed in triplicate. The SAND gene expression was stable among flower samples and thus chosen as internal standard for normalization. Primers used for qRT–PCR detection were previously described18 and their sequences are provided in Supplementary Table 6.
Phylogenetic dN/dS analysis
Coding sequences were aligned with MUSCLE49 and phylogenies were built using PhyML50 using the generalized time-reversible (GTR) model with gamma distribution (four rate classes of sites with optimized alpha) via the Seaview software (http://pbil.univ-lyon1.fr/)51. PAML (codeml) (http://abacus.gene.ucl.ac.uk/software/paml.html)52 and Fitmodel53 were applied to the coding-sequence alignments and phylogenetic trees. All the analyses were run both with a trimmed alignment (404 over 560 codons) where the most variable sites, mainly in the 5′ and 3′ regions of the coding sequences, were suppressed by GBlocks54,55 and with a longer alignment where all but the gap-containing sites were conserved (457 over 560 codons). We ran ‘Branch model’, ‘Site model’, ‘Clade model’, ‘Branch-site’ and ‘Free-ratio’ analyses following codeml standard procedures. We compared nested models using the likelihood ratio test framework to test statistical differences in dN/dS. We ran Fitmodel M2a+S1 (with ω1<ω2<ω3 and switch between selection regimes allowed) and MX+S1 (with ω0≤1; 1<ω1≤1.5; ω2>1.5 and switch between selection regimes allowed) as an alternative ‘Branch-site’ analysis and M2a (no switch between selection regimes allowed) as a null model to perform a likelihood ratio test53. All results and associated statistical tests are available in Supplementary Note 1 and Supplementary Table 2.
Homology modelling
All Modeller and Autodock calculations were performed using the computing facilities of the CEA-DSV (cluster Gabriel) at Saclay.
Three-dimensional models of A. thaliana CYP98A8/9 isoforms deprived of their membrane spanning domain (residues 24 to 491 for 98A8, and residues 24 to 485 for 98A9) were built using Modeller9v12 (refs 56, 57) and the crystal structures of CYP2C9 (PDB 1OG2), CYP2A6 (PDB 2PG6), CYP2D6 (PDB 2F9Q), CYP2B4 (PDB 3MVR), and CYP2C5 (PDB 1DT6) as templates. The selection of the template structures for CYP98A8/9 model rebuilding followed four criteria: the best similarity according to PDB-Blast, the highest alignment length, the atomic resolution and ligand-free structures for getting undeformed states. These mammalian CYPs exhibited rather low sequence identities with AtCYP98A8 isoforms (24 to 25%), but the three-dimensional structures are well-aligned by MUSTANG algorithm58, and the resulting alignment was consistent with that obtained with MAFFT-L-INS-I algorithm59. The validated sequence alignment used as input for Modeller is available in Supplementary Fig. 10.
For each isoform, two runs of 100 models were performed with a further loop refinement protocol, and the generated models sorted by the MODELLER objective function were evaluated by their discrete optimized protein energy and GA341 scores calculated by Modeller. For each isoform, the five best models (corresponding to the lowest discrete optimized protein energy scores) issued from each run were pooled and submitted to the online metaserver SAVES (structural analysis and verification server (http://services.mbi.ucla.edu/SAVES), and finally to the QMEAN scoring function60 server61 for model quality assessment. The final model was the best one according to a good compromise between the scores calculated by the SAVES server scoring programs and the QMEAN value. As a result, the selected AtCYP98A8 and AtCYP98A9 models had a QMEAN score equal to 0.701 and 0.689, respectively, which are good scores when compared to the QMEAN scores of individual PDB templates.
Docking experiments
The protein structures generated by Modeller were first stripped of all hydrogen atoms, and then atom charges and hydrogen atoms were added to the protein with the UCSF Chimera package (www.cgl.ucsf.edu/chimera)62 using AMBER ff99SB parameters. The first parameters applied for the heme (defined as FeIII protoporphyrin IX) were obtained from Oda et al.63, and in the docking experiments with S-Naringenin (Nar) the heme cofactor was defined as the highly reactive intermediate compound I (FeIV=O)+ by using the parameters (geometry and atom charges) of an AMBER-compatible heme model developed by Shahrokh et al.64. The atom charges of the proximal thiolate were taken from the same work.
Molecular docking experiments with the flavonoid S-naringenin (Nar) at the active site were performed using AutoDock 4 (release 4.2.6) in the semi-flexible mode, and prepared with AutoDock Tools65. The ligand molecule, Nar, was parametrized under Maestro 9.2 (www.schrödinger.com) molecular modelling suite and the structure saved under MOL2 format as input files for AutoDock. Partial charges were assigned using OPLS 2005 force field. There is only one rotatable bond in Nar, but several conformations of the non-aromatic ring have been generated as starting point for docking to allow better conformational sampling under Autodock 4, since the algorithm cannot handle ring flexibility. The MOL2 format files, created in Chimera for the receptor and Maestro for the ligand, were converted into the PDBQT format file by AutoDockTools, which merges all nonpolar hydrogen atoms to the carbon atoms they are bonded to. The receptor was kept rigid.
The docking box, in which grid maps were computed using program AutoGrid65, included the active site with the iron-protoporphyrin group on one edge, and the whole distal moiety of the enzyme, including access channels and protein surface, to allow a large sampling of potential poses. The grid built by AutoGrid included 100, 70 and 86 points in x, y and z directions, with a grid spacing of 0.37Å to allow a good compromise between resolution of the explored volume and the size of the binding area. For each Nar conformer, 500 independent runs were performed using the Lamarckian genetic algorithm66. The default settings were used for all other parameters.
The poses were assigned a score calculated by Autodock that can be considered as an estimated free energy of ligand binding (indicative of binding affinity), then clustered as a function of the closeness of their positions and conformations with root mean square deviation set at 2.0 Å, and finally ranked by their binding score (for the best pose in the cluster). The resulting histograms of 500 poses of Nar docked into AtCYP98A8 and AtCYP98A9 are visible in the Supplementary Fig. 11. Remarkably, whereas the docking computational conditions were identical for both isoforms, the solutions found for AtCYP98A9 are gathered in the same cluster, within 2 Å of root mean square deviation of clustering, while the histogram of AtCYP98A8 displays a more scattered profile. The best clusters, corresponding to the lowest (that is, the most negative) energetic binding scores, were well-resolved in the histogram of scores for AtCYP98A8 but corresponded to non-productive metabolism positions. All clusters under −4 kcal mol−1 were independently investigated for their consistency with productive position for 3′-hydroxylation reaction.
Data availability
The data that support the findings of this study are available from the corresponding author upon request. CYP98A8 sequence data from E. syriacum and C. tenella have been deposited in GenBank with accession codes KX754460 and KX754461.
Additional information
How to cite this article: Liu, Z. et al. Evolutionary interplay between sister cytochrome P450 genes shapes plasticity in plant metabolism. Nat. Commun. 7, 13026 doi: 10.1038/ncomms13026 (2016).
References
Ohno, S. Evolution by Gene Duplication Springer Verlag (1970).
Lynch, M. & Conery, J. S. The evolutionary fate and consequences of duplicate genes. Science 290, 1151–1155 (2000).
Davison, A. J, Benko, M. & Harrach, B. Genetic content and evolution of adenoviruses. J. Gen. Virol. 84, 2895–2908 (2003).
Roth, C. et al. Evolution after gene duplication: models, mechanisms, sequences, systems, and organisms. J. Exp. Zool. B Mol. Dev. Evol. 308, 58–73 (2007).
Innan, H. & Kondrashov, F. The evolution of gene duplications: classifying and distinguishing between models. Nat. Rev. Genet. 11, 97–108 (2010).
Hughes, A. L. The evolution of functionally novel proteins after gene duplication. Proc. Biol. Sci. 256, 119–124 (1994).
Force, A. et al. Preservation of duplicate genes by complementary, degenerative mutations. Genetics 151, 1531–1545 (1999).
Stoltzfus, A. On the possibility of constructive neutral evolution. J. Mol. Evol. 49, 169–181 (1999).
Des Marais, D. L. & Rausher, M. D. Escape from adaptive conflict after duplication in an anthocyanin pathway gene. Nature 454, 762–765 (2008).
Hittinger, C. T. & Carroll, S. B. Gene duplication and the adaptive evolution of a classic genetic switch. Nature 449, 677–681 (2007).
Deng, C., Cheng, C. H., Ye, H., He, X. & Chen, L. Evolution of an antifreeze protein by neofunctionalization under escape from adaptive conflict. Proc. Natl Acad. Sci. USA 107, 21593–21598 (2010).
Henderson, B. & Martin, A. C. Protein moonlighting: a new factor in biology and medicine. Biochem. Soc. Trans. 42, 1671–1678 (2014).
Copley, S. D. An evolutionary perspective on protein moonlighting. Biochem. Soc. Trans. 42, 1684–1691 (2014).
Aury, J. M. et al. Global trends of whole-genome duplications revealed by the ciliate Paramecium tetraurelia. Nature 444, 171–178 (2006).
He, X. & Zhang, J. Rapid subfunctionalization accompanied by prolonged and substantial neofunctionalization in duplicate gene evolution. Genetics 169, 1157–1164 (2005).
Beilstein, M. A. et al. Evolution of the telomere-associated protein POT1a in Arabidopsis thaliana is characterized by positive selection to reinforce protein-protein interaction. Mol. Biol. Evo. 32, 1329–1341 (2015).
Lynch, M. & Walsh, B. The Origins of Genome Architecture Sinauer Associates Sunderland (2007).
Matsuno, M. et al. Evolution of a novel phenolic pathway for pollen development. Science 325, 1688–1692 (2009).
Schoch, G. et al. CYP98A3 from Arabidopsis thaliana is a 3'-hydroxylase of phenolic esters, a missing link in the phenylpropanoid pathway. J. Biol. Chem. 276, 36566–36574 (2001).
Franke, R. et al. The Arabidopsis REF8 gene encodes the 3-hydroxylase of phenylpropanoid metabolism. Plant J. 30, 33–45 (2002).
Weng, J. K. & Chapple, C. The origin and evolution of lignin biosynthesis. New Phytol. 187, 273–285 (2010).
Xu, J. et al. ABORTED MICROSPORES acts as a master regulator of pollen wall formation in Arabidopsis. Plant Cell 26, 1544–1556 (2014).
Beilstein, M. A., Nagalingum, N. S., Clements, M. D., Manchester, S. R. & Mathews, S. Dated molecular phylogenies indicate a Miocene origin for Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 107, 18724–18728 (2010).
Cheng, S. et al. The Tarenaya hassleriana genome provides insight into reproductive trait and genome evolution of crucifers. Plant Cell 25, 2813–2830 (2013).
Ming, R. et al. The draft genome of the transgenic tropical fruit tree papaya (Carica papaya Linnaeus). Nature 452, 991–996 (2008).
McDonald, J. H. & Kreitman, M. Adaptive protein evolution at the Adh locus in Drosophila. Nature 351, 652–654 (1991).
Belmonte, M. F. et al. Comprehensive developmental profiles of gene activity in regions and subregions of the Arabidopsis seed. Proc. Natl Acad. Sci. USA 110, E435–E444 (2013).
Bell-Lelong, D. A., Cusumano, J. C., Meyer, K. & Chapple, C. Cinnamate-4-hydroxylase expression in Arabidopsis. Regulation in response to development and the environment. Plant Physiol. 113, 729–738 (1997).
Buer, C. S. & Muday, G. K. The transparent testa 4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. Plant Cell 16, 1191–1205 (2004).
Buer, C. S., Imin, N. & Djordjevic, M. A. Flavonoids: new roles for old molecules. J. Integr. Plant Biol. 52, 98–111 (2010).
Deshpande, S. S., Cheryan, M. & Salunkhe, D. K. Tannin analysis of food products. Crit. Rev. Food Sci. Nutr. 24, 401–449 (1986).
Lepiniec, L. et al. Genetics and biochemistry of seed flavonoids. Annu. Rev. Plant Biol. 57, 405–430 (2006).
Mierziak, J., Kostyn, K. & Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 19, 16240–16265 (2014).
Doughty, J., Aljabri, M. & Scott, R. J. Flavonoids and the regulation of seed size in Arabidopsis. Biochem. Soc. Trans. 42, 364–369 (2014).
Robinson, S. J. et al. An archived activation tagged population of Arabidopsis thaliana to facilitate forward genetics approaches. BMC Plant Biol. 9, 101 (2009).
Weng, J. K., Mo, H. & Chapple, C. Over-expression of F5H in COMT-deficient Arabidopsis leads to enrichment of an unusual lignin and disruption of pollen wall formation. Plant J. 64, 898–911 (2010).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Liu, Y. G. & Chen, Y. High-efficiency thermal asymmetric interlaced PCR for amplification of unknown flanking sequences. Biotechniques 43, 649–656 (2007).
Beilstein, M. A., Al-Shehbaz, I. A. & Kellogg, E. A. Brassicaceae phylogeny and trichome evolution. Am. J. Bot. 93, 607–619 (2006).
Lyons, E. & Freeling, M. How to usefully compare homologous plant genes and chromosomes as DNA sequences. Plant J. 53, 661–673 (2008).
Nour-Eldin, H. H., Hansen, B. G., Norholm, M. H., Jensen, J. K. & Halkier, B. A. Advancing uracil-excision based cloning towards an ideal technique for cloning PCR fragments. Nucleic Acids Res. 34, e122 (2006).
Höfer, R. et al. Geraniol hydroxylase and hydroxygeraniol oxidase activities of the CYP76 family of cytochrome P450 enzymes and potential for engineering the early steps of the (seco)iridoid pathway. Metab. Eng. 20, 221–232 (2013).
Urban, P., Mignotte, C., Kazmaier, M., Delorme, F. & Pompon, D. Cloning, yeast expression, and characterization of the coupling of two distantly related Arabidopsis thaliana NADPH-cytochrome P450 reductases with P450 CYP73A5. J. Biol. Chem. 272, 19176–19186 (1997).
Routaboul, J. M. et al. Flavonoid diversity and biosynthesis in seed of Arabidopsis thaliana. Planta 224, 96–107 (2006).
Hirai, M. Y. et al. Toward genome-wide metabolotyping and elucidation of metabolic system: metabolic profiling of large-scale bioresources. J. Plant Res. 123, 291–298 (2010).
Matsuda, F. et al. AtMetExpress development: a phytochemical atlas of Arabidopsis development. Plant Physiol. 152, 566–578 (2010).
Stangeland, B. & Salehian, Z. An improved clearing method for GUS assay in Arabidopsis endosperm and seeds. Plant. Mol. Biol. Rep. 20, 107–114 (2002).
Zimmermann, P., Hirsch-Hoffmann, M., Hennig, L & Gruissem, W. GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol. 136, 2621–2632 (2004).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Guindon, S. et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59, 307–321 (2010).
Gouy, M., Guindon, S. & Gascuel, O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 27, 221–224 (2010).
Yang, Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 24, 1586–1591 (2007).
Guindon, S., Rodrigo, A. G., Dyer, K. A. & Huelsenbeck, J. P. Modeling the site-specific variation of selection patterns along lineages. Proc. Natl Acad. Sci. USA 101, 12957–12962 (2004).
Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 17, 540–552 (2000).
Talavera, G. & Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 56, 564–577 (2007).
Sali, A. & Blundell, T. L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234, 779–815 (1993).
Webb, B. & Sali, A. Comparative protein structure modeling using MODELLER. Curr. Protoc. Bioinformatics 47, 5.6.1–5.6.32 (2014).
Konagurthu, A. S., Whisstock, J. C., Stuckey, P. J. & Lesk, A. M. MUSTANG: a multiple structural alignment algorithm. Proteins 64, 559–574 (2006).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Benkert, P., Tosatto, S. C. & Schomburg, D. QMEAN: A comprehensive scoring function for model quality assessment. Proteins 71, 261–277 (2008).
Benkert, P., Kunzli, M. & Schwede, T. QMEAN server for protein model quality estimation. Nucleic Acids Res. 37, W510–W514 (2009).
Pettersen, E. F. et al. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Oda, A., Yamaotsu, N. & Hirono, S. New AMBER force field parameters of heme iron for cytochrome P450s determined by quantum chemical calculations of simplified models. J. Comput. Chem. 26, 818–826 (2005).
Shahrokh, K., Orendt, A., Yost, G. S. & Cheatham, T. E. 3rd Quantum mechanically derived AMBER-compatible heme parameters for various states of the cytochrome P450 catalytic cycle. J. Comput. Chem. 33, 119–133 (2012).
Morris, G. M. et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 30, 2785–2791 (2009).
Fuhrmann, J., Rurainski, A., Lenhof, H. P. & Neumann, D. A new Lamarckian genetic algorithm for flexible ligand-receptor docking. J. Comput. Chem. 31, 1911–1918 (2010).
Acknowledgements
We are grateful to Clint Chapple (Purdue university, USA) for providing the pCC0996 vector. Z.L. is grateful to the China Scholarship Council and the Région Alsace for co-funding a PhD scholarship. D.W.-R. and H.R. acknowledge the support of the Agence Nationale pour la Recherche to the PHENOWALL ANR-10-BLAN-1528 project, and of the Freiburg Institute for Advanced Studies (FRIAS) and the University of Strasbourg Institute for Advanced Study (USIAS) to the METABEVO project. F.A. acknowledges Laura Dhers (PhD student, Université Paris Descartes) for help in model structures set-up and the staff of the computing facility of the Commissariat à l'Energie Atomique (CEA/DSV/GIPSI, Saclay) for help and access to the national cluster Gabriel. A portion of this work was supported by a grant (MCB 1409251) from the National Science Foundation of the United States to M.A.B.
Author information
Authors and Affiliations
Contributions
Z.L. performed most of the experiments and contributed to molecular evolution analyses supervised by R.T., to metabolic profiling together with R.L., S.B.-M., T.T. and H.R., and to project design. R.T. performed PAML and Fitmodel analyses and E.S.F. and M.A.B. performed McDonald–Kreitman tests. Homology modelling and docking analyses were performed by F.A. and G.J., molecular characterization of Brassicaceae lineage II and III CYP98 members was performed by E.S.F. and M.A.B., and tests for flavonoid metabolism and site-directed mutagenesis were performed by H.R. H.R. ensured project design and supervision, and performed data analysis and visualization. D.W.-R. designed the project and supervised the discussions. Z.L., H.R. and D.W.-R. wrote the initial draft, subsequently edited by Z.L., E.S.F., M.A.B., R.T., H.R. and D.W.-R.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-11, Supplementary Tables 1-6 and Supplementary Note 1 (PDF 1832 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Liu, Z., Tavares, R., Forsythe, E. et al. Evolutionary interplay between sister cytochrome P450 genes shapes plasticity in plant metabolism. Nat Commun 7, 13026 (2016). https://doi.org/10.1038/ncomms13026
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms13026
This article is cited by
-
Diversity and evolution of cytochromes P450 in stramenopiles
Planta (2019)
-
A phenol-enriched cuticle is ancestral to lignin evolution in land plants
Nature Communications (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.