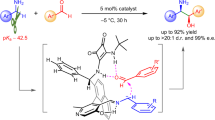
Abstract
Several examples on Pd-catalysed carbonylation of methyl C(sp3)–H bonds with gaseous CO via Pd(II)/Pd(0) catalysis have been reported. However, methylene C(sp3)–H carbonylation remains a great challenge, largely due to the lack of reactivity of C–H bonds and the difficulty in CO migratory insertion. Herein, we report the stereoselective alkoxycarbonylation of both methyl and methylene C(sp3)–H bonds with alkyl chloroformates through a Pd(II)/Pd(IV) catalytic cycle. A broad range of aliphatic carboxamides and alkyl chloroformates are compatible with this protocol. In addition, this process is scalable and the directing group could be easily removed under mild conditions with complete retention of configuration.
Similar content being viewed by others
Introduction
Over the past few decades, Pd-catalysed C–H functionalization has emerged as a powerful tool for the direct conversion of ubiquitous C–H bonds into diverse functional groups1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16. Among various C–H functionalization reactions, the direct alkoxycarbonylation of C–H bonds is particularly valuable17,18,19,20,21, since the resulting products, esters, are among the most important functional groups that appear commonly in agrochemicals, fine chemicals, natural products and pharmaceuticals.
Although Pd-catalysed carbonylation of aromatic C–H bonds has been extensively investigated, the direct carbonylation of aliphatic C–H bonds is limited and still represents a tremendous challenge in organic synthesis17,18,19,20,21. Pd-catalysed carbonylation of methyl C(sp3)–H bonds of aliphatic amides or amines with CO for the synthesis of succinimides or lactams has been achieved recently22,23,24,25,26,27. Generally, these reactions proceed through a Pd(II)/Pd(0) catalytic cycle. While these elegant methods are efficient to introduce carbonyl groups and have greatly enriched the reaction scope, the use of CO is still relatively inconvenient on laboratory-scale due to its gaseous form, toxic nature and flammability. In addition, the carbonylation reactions were limited to those functionalizing methyl C(sp3)–H bonds (Fig. 1a)22,23,24,25,26,27. The analogous carbonylation of methylene C(sp3)–H bonds, which are more inert and sterically hindered than methyl C(sp3)–H bonds, remains unexplored28,29.
Pd-catalysed alkoxycarboxylation of C(sp2)–H bonds with other carbonyl reagents, such as potassium oxalate monoester30, DMF31, formates32, azodicarboxylates33,34, α-keto esters35, glyoxylates36 and oxaziridine37 has been reported. Ru-catalysed alkoxycarbonylation of 2-arylpyridines with alkyl chloroformates was disclosed by Kakiuchi and co-workers38. Inspired by these excellent precedents and based on our recent work on Pd-catalysed C(sp3)–H functionalization39,40,41, we were eager to develop the catalytic carbonylation of methylene C(sp3)–H bonds with less toxic, more easy to handle and readily available carbonyl reagents.
Herein, we report the stereoselective and site-selective alkoxycarbonylation of unactivated C(sp3)–H bonds with alkyl chloroformates through a Pd(II)/Pd(IV) catalytic cycle (Fig. 1b). This reaction is environmentally friendly and operationally simple. A broad range of aliphatic carboxamides and alkyl chloroformates are compatible with this protocol. In addition, this process is scalable and the directing group could be easily removed under mild conditions with complete retention of configuration, thus providing a convenient strategy for the stereoselective synthesis of orthogonally protected aspartic acid derivatives42,43,44,45. Compared with the well-established C–H carbonylation with CO via Pd(II)/Pd(0) catalysis, the direct alkoxycarbonylation of unactivated C(sp3)–H bonds through a Pd(II)/Pd(IV) catalytic cycle provides a new mode of reaction and might offer a distinct platform for reaction development.
Results
Proof of concept on methylene C(sp3)–H alkoxycarbonylation
We began our investigation by using N-phthaloyl phenylalanine derivative 1a bearing an 8-aminoquinoline (AQ) auxiliary as a model substrate. This auxiliary was first introduced by Daugulis and has been proved to be effective in the direct functionalization of methylene C(sp3)–H bonds46,47,48,49,50. We first explored the carbonylation with carbon monoxide through the traditional Pd(II)/Pd(0) pathway. Previously, we have found that the reaction of N-phthaloyl phenylalanine derivative 1a and stoichiometric Pd(OAc)2 could form the stable palladacycle I in MeCN (Fig. 2a)51,52. However, when we treated palladacycle I with carbon monoxide under various conditions, no desired carbonylation product 3ab was observed. Complex II with CO coordinated as an L-type ligand was obtained as a pale yellow solid in 80% yield (Fig. 2b). This complex showed unexpected resistance to migratory insertion under various conditions, and proved stable to air and moisture, withstanding shelf storage without noticeable decomposition. The coordination of CO as a neutral ligand without migratory insertion was confirmed by the characteristic infrared absorption of terminally coordinated CO ligand (2095, cm−1, Supplementary Fig. 2), and, the ease formation of complex III via ligand exchange with pyridine, which was characterized by X-ray crystallography (Fig. 2d). It is noteworthy that complex III could also be generated via the reaction of 1a with 1 equivalent of Pd(OAc)2 in a mixture of DCE and pyridine (see Supplementary Methods for details). Moreover, the reaction of 1a with stoichiometric Pd(OAc)2 under 1 atm CO could also give complex II in 80% yield without the detection of any carbonylated product 3ab (Fig. 2c). Thus, the carbonylation of β-methylene C(sp3)–H bonds of 1a through Pd(II)/Pd(0) was unfeasible due to the difficulty in CO migratory insertion and subsequent reductive elimination53,54.
(a) Synthesis of palladacycle I. (b) Stoichiometric reaction of palladacycle I with carbon monoxide. (c) Synthesis of CO-coordinated Pd(II) complex II. (d) Structure confirmation of complex II via the transformation to complex III. The structure of complex III was unambiguously confirmed by single-crystal X-ray diffraction. (e) Stoichiometric reaction of palladacycle I with ClCO2R. The structure of compounds 3a and 4b was confirmed by single-crystal X-ray diffraction.
It has been proven that high-valent Pd(IV) species undergo facile reductive elimination55,56,57,58,59. Therefore, we speculated that the use of alkyl chloroformates as carbonylation reagent might enable the desired alkoxycarbonylation via oxidative addition of palladacycle I to form a highly reactive Pd(IV) intermediate IV, which could then undergo reductive elimination to give the corresponding ester (Scheme 1, Pd(II)/Pd(IV) pathway). To our delight, treatment of complex I with 3 equiv. of ClCO2Et (2ab) or ClCO2Me (2b) in the presence of 2 equiv. of silver carbonate gives the expected alkoxycarbonylation product 3a and 4b in 25% and 18% yield, respectively. The relative and absolute stereochemistry of 3a and 4b was unambiguously determined by X-ray crystallography (Fig. 2e). Inspired by this promising result, we next sought to identify suitable reaction conditions to render this reaction catalytically (Table 1). When 1a was treated with 10 mol% Pd(OAc)2, 3 equiv. of ClCO2Et and 2 equiv. Ag2CO3 in DCM, the desired product 3a was obtained in 40% yield, along with trace of undesired β-lactam 3aa generated by the competitive intramolecular C–N bond reductive elimination (entry 1). Toluene was found to be the ideal solvent for this transformation (entry 7, 65% yield). Further screening of additives then established that the addition of I2 could significantly improved the efficiency and 3a was obtained in 76% isolated yield (entry 11). It is worth noting that the alkoxycarbonylation reaction was quite sensitive to the amount of Ag2CO3. Attempts to lower the Ag2CO3 loading led to the inhibition of the desired reaction and the competitive intramolecular C–N bond reductive elimination occured predominantly (entry 12, 3a, <5%; 3aa, 30%).
Substrate scope of methylene C(sp3)–H alkoxycarbonyaltion
With the optimal reaction conditions in hand, the scope of this alkoxycarbonylation reaction was investigated (Fig. 3). The reaction was found to be compatible with a broad range of phenylalanine derivatives with various electron-donating and electron-withdrawing substituents (3b–3q). Various functional groups, such as methoxy (3e–3g), trifluoromethyl (3h), acetyl (3i), methoxycarbonyl (3j), fluoro (3k and 3l), chloro (3m) and bromo (3n) were tolerated, furnishing the desired products in moderate to good yields. The tolerance of halides was particularly noteworthy since such substituents could serve as versatile handles for further elaboration via cross-coupling. It is noteworthy that arylalanine derivatives (1b–1q) were prepared by arylation of the alanine derivative (5a) using our previsouly established conditions40. Therefore, this protocol also showcases the synthesis of chiral aspartic acid derivatives via a two-step C–H functionalization sequence. Importantly, the alkoxycarbonylation of aliphatic secondary C(sp3)–H bonds could also be achieved when 0.2 equiv. of succinic anhydride was included (3r-3w). Alkoxycarbonylation of sterically hindered L-leucine derivative containing adjacent secondary alkyl group occurred smoothly under a slightly higher temperature (3t, 50%). The alkoxycarbonylation reaction was found to be highly diastereoselective, furnishing a single diastereoisomoer as the sole product. The relative and absolute stereochemistry of 3a, 3d, 3k and 4b, was unambiguously determined by X-ray diffraction, and all other alkoxycarbonylation products were assigned analogically. The trans orientation of the N-phthaloyl group and the newly incorporated alkoxycarbonyl group was consistent with the proposed stereochemical model and previous reports40,41,42,43,44,45,51,52. In addition, the more remote γ-methyl C(sp3)–H bond was also reactive, provided that no reactive β–C–H bonds were present. The alkoxycarbonylation of L-tert-Leucine (3u), L-isoleucine (3v) and L-vlaine (3w) proceeded effectively, albeit affording the products in reduced yields.
The scope of the alkyl chloroformates coupling partners was examined subsequently (Fig. 4). The reaction was found to be compatible with a variety of simple and more complex chloroformates. Methoxycarbonylation of phenylanine derivative proceeded smoothly to provide product 4b in 72% yield. A number of linear alkyl chloroformates gave the corresponding products in good yields (4b–4e). Interestingly, the more sterically hindered branched-alkyl chloroformates, such as iPr (2f), iBu (2g), and cyclopentyl (2h) were more reactive, giving the corresponding products in higher yields (4f–4h, 82–96% yield). The synthetic potential of this alkoxycarbonylation strategy was further demonstrated by the effective reaction with more complex chloroformates, such as menthyl chloroformate (2i) and Fmoc-Cl (2j).
Substrate scope of methyl C(sp3)–H alkoxycarbonyaltion
Next, we sought to investigate whether the alkoxycarbonylation protocol amendable to methyl C(sp3)–H bonds. Gratifyingly, the alkoxycarbonylation occurred smoothly to a variety of aliphatic carboxamides bearing β-methyl C–H bonds with a slightly modified conditions: 10 mol% Pd(TFA)2, 2.0 equiv. Ag2CO3, 1.0 equiv. Na3PO4 and 3.0 equiv. ClCO2Me in toluene at 120 °C. As shown in Fig. 5, N-phthaloyl alanine derivative 5a reacted effeciently with ClCO2Me in the absence of sodium phosphate, affording the orthogonally protected aspartic acid 6a in 71% yield. Aliphatic carboxamides bearing either linear chains or cyclohexyl were also compatible with these new conditions (6b–6f). A wide range of functional groups, such as ester (6g), ethers (Bn-, 6h and 6l; Et-, 6k), alkene (6i) and alkyne (6j), could also be readily alkoxycarbonylated. The reactions were also tolerant of a number of aryl groups at the α, β- and δ-positions of the carboxamides (6m–6o and 6e). It should be noted that the broad functional group tolerance highlights the synthetic potential of this protocol in late-stage modification and total synthesis of complex molecules60.
Synthetic potential
To further demonstrate the synthetic potential of this reaction, the reaction was conducted in gram scale (Fig. 6). We were pleased to find that the treatment of 3.0 mmol 1a with ethyl chloroformate gave the corresponding ethoxycarbonylation product 3a in 74% isolated yield (1.10 g). The competitive intramolecular C–N bond reductive elimination product 3aa was also produced in 12% yield when the reaction was scaled up. The desired product 3a was obtained without any racemization. Moreover, we also found that no epimerization of 3a has been observed upon prolonged heating under the reaction conditions (Supplementary Figs 8–10).
The ability to easily remove the directing group from the final product is crucial for synthetic applications of this reaction. Previously, we reported that 2-pyridinylisopropyl bidentate auxiliary introduced by us could be removed through a nitrosylation/hydrolysis sequence with a mixture of NaNO2/AcOH/Ac2O (refs 39, 40). Baudoin and co-workers has improved the procedure by using NOBF4 as a nitrosation agent and pyridine at low temperature61,62. We envisioned that novel process could also be applied to the removal of AQ. As expected, the corresponding carboxylic acid 7 was obtained in 62% yield without further optimization. Following esterification, the corresponding methyl ester 8 was obtained in 91% yield with the retention of configuration (Scheme 3, see Supplementary Methods and Supplementary Fig. 61 for details). It is worth noting that all of the alkoxycarbonylation reactions were operationally simple, without the need for an inert-atmosphere or rigorously moisture-free conditions.
Mechanistic investigations
To shed light on the mechanism, several experiments were performed (Fig. 7). First, kinetic isotopic effect (KIE) experiments were conducted by treatment of compound 1a and its deuterated analogue 1a-d2 under the standard reaction conditions for 10 min. A kH/kD value of 1.5 was obtained in a competitive reaction and 1.7 in parallel reactions on the basis of 1H nuclear magnetic resonance (NMR) analysis (Fig. 7a), which is indicative of a secondary kinetic isotope effect. This result also suggests that the cleavage of C–H is not the rate-determining step of the reaction.
Second, a stoichiometric reaction of complex I with 3 equivalents of ClCO2Et (2a) under the optimized reaction conditions was performed, and the alkoxycarbonylated product 3a was obtained in 40% yield. However, β-lactam 3aa was produced in 48% yield and no desired product 3a was observed when complex I was treated with iodine in the absence of silver carbonate (Fig. 7b). These results clearly indicated that the addition of silver carbonate was crucial for the success of this transformation. Although the exact role of the silver salt may simply be a halide scavenger63,64,65, it is also proposed to form a bimetallic complex with palladium, which might be important for the reaction24,27,66.
Finally, we found that palladacycle I was a viable precatalyst for the alkoxycarbonylation of 1a, providing the desired product in 70% yield, which was comparable with the result under the standard conditions (Fig. 7c).
Discussion
In conclusion, we have developed a new protocol for the direct alkoxycarbonylation of both methylene and methyl C(sp3)–H bonds through a Pd(II)/Pd(IV) catalytic cycle. A variety of operationally simple and readily available alkyl chloroformates were used as carbonyl sources. The reaction proceeded with high functional compatibility. Furthermore, this efficient and stereoselective protocol to access orthogonally protected chiral aspartic acid derivatives may find applications in the synthesis of complex molecules. Compared with the well-established C–H carbonylation with CO via Pd(II)/Pd(0) catalysis, the direct alkoxycarbonylation of unactivated C–H bonds through a Pd(II)/Pd(IV) catalytic cycle provides a new mode of reaction and might offer a distinct platform for reaction development. Further studies toward the application of this new strategy to other reaction systems are currently underway.
Methods
General methods
For NMR spectra, high-performance liquid chromatography (HPLC) data, and X-ray analysis of compounds in this manuscript and detailed experimental procedures, see Supplementary Figs 1–67, Supplementary Tables 1–12 and Supplementary Methods. See Supplementary Datasets 1–6 for X-ray CIF files of compounds III, 3a, 3d, 3k, 4b and 6a (CCDC 1446624, 1487147, 1486639, 1486599, 1446623, 1475241).
General procedure for secondary C–H alkoxycarbonylation
To a 50 ml Schlenk tube, were added 1 (0.15 mmol), Pd(OAc)2 (3.5 mg, 0.015 mmol), Ag2CO3 (82.7 mg, 0.3 mmol), I2 (38.0 mg, 1.0 equiv.), ClCO2R (0.45 mmol, 3.0 equiv.) and toluene (2.0 ml). The tube was sealed under air. The mixture was stirred at room temperature for 5 min then heated at 120 °C for 16 h. After cooling to room temperature, the reaction mixture was diluted with EtOAc (10 ml) and filtered through a pad of Celite. After concentration in vacuo, the crude reaction mixture was purified by silica gel flash chromatography.
General Procedure for Primary C–H Alkoxycarbonylation
To a 50 ml Schlenk tube, were added 1 (0.15 mmol), Pd(OTFA)2 (5.0 mg, 0.015 mmol), Ag2CO3 (82.7 mg, 0.3 mmol), Na3PO4 (49.0 mg, 0.3 mmol), ClCO2Me (0.45 mmol, 3.0 equiv.) and toluene (2.0 ml). The tube was sealed under air. The mixture was stirred at room temperature for 5 min then heated at 120 °C for 20 h. After cooling to room temperature, the reaction mixture was diluted with EtOAc (10 ml) and filtered through a pad of Celite. After concentration in vacuo, the crude reaction mixture was purified by silica gel flash chromatography.
Data availability
The X-ray crystallographic structures for compounds III, 3a, 3d, 3k, 4b, 6a reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), with the accession codes CCDC 1446624, 1487147, 1486639, 1486599, 1446623, 1475241 (http://www.ccdc.cam.ac.uk/data_request/cif). The authors declare that all other relevant data supporting the findings of this study are available within the article and its Supplementary Information files.
Additional information
How to cite this article: Liao, G. et al. Stereoselective alkoxycarbonylation of unactivated C(sp3)–H bonds with alkyl chloroformates via Pd(II)/Pd(IV) catalysis. Nat. Commun. 7:12901 doi: 10.1038/ncomms12901 (2016).
References
Davies, H. M. L. & Manning, J. R. Catalytic C–H functionalization by metal carbenoid and nitrenoid insertion. Nature 451, 417–424 (2008).
Davies, H. M. L., Du Bois, J. & Yu, J.-Q. C–H functionalization in organic synthesis. Chem. Soc. Rev. 40, 1855–1856 (2011).
Ackermann, L. Carboxylate-assisted transition-metal-catalyzed C–H bond functionalizations: mechanism and scope. Chem. Rev. 111, 1315–1345 (2011).
Hartwig, J. F. Regioselectivity of the borylation of alkanes and arenes. Chem. Soc. Rev. 40, 1992–2002 (2011).
Huang, Z., Lim, H., Mo, F., Young, M. C. & Dong, G. Transition metal-catalyzed ketone-directed or mediated C–H functionalization. Chem. Soc. Rev. 44, 7764–7786 (2015).
Wencel-Delord, J., Dröge, T., Liu, F. & Glorius, F. Towards mild metal-catalyzed C–H bond activation. Chem. Soc. Rev. 40, 4740–4761 (2011).
Song, G., Wang, F. & Li, X. C–C, C–O and C–N bond formation via Rhodium(III)-catalyzed oxidative C-H activation. Chem. Soc. Rev. 41, 3651–3678 (2012).
Jazzar, R., Hitce, J., Renaudat, A., Sofack-Kreutzer, J. & Baudoin, O. Functionalization of organic molecules by transition-metal-catalyzed C(sp3 )–H activation chemistry. Chem. Eur. J. 16, 2654–2672 (2010).
Daugulis, O., Do, H.-Q. & Shabashov, D. Palladium- and copper-catalyzed arylation of carbon–hydrogen bonds. Acc. Chem. Res. 42, 1074–1086 (2009).
Engle, K. M., Wu, H.-C. & Yu, J.-Q. Weak coordination as a powerful means for developing broadly useful C–H functionalization reactions. Acc. Chem. Res. 45, 788–802 (2012).
Lyons, T. W. & Sanford, M. S. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev. 110, 1147–1169 (2010).
Cho, S. H., Kim, J. Y., Kwak, J. & Chang, S. Recent advances in the transition metal-catalyzed twofold oxidative C–H bond activation strategy for C–C and C–N bond formation. Chem. Soc. Rev. 40, 5068–5083 (2011).
Brückl, T., Baxter, R. D., Ishihara, Y. & Baran, P. S. Innate and guided C–H functionalization logic. Acc. Chem. Res. 45, 826 (2012).
Li, B.-J. & Shi, Z.-J. From C(sp2) -H to C(sp3) -H: systematic studies on transition metal-catalyzed oxidative C -C formation. Chem. Soc. Rev. 41, 5588 (2012).
Engle, K. M. & Yu, J.-Q. Developing ligands for palladium(II)-catalyzed C–H functionalization: intimate dialogue between ligand and substrate. J. Org. Chem. 78, 8927–8955 (2013).
Zhang, X.-S., Chen, K. & Shi, Z.-J. Transition metal-catalyzed direct nucleophilic addition of C–H bonds to carbon–heteroatom double bonds. Chem. Sci. 5, 2146–2159 (2014).
Yang, L. & Huang, H. Transition-metal-catalyzed direct addition of unactivated C–H bonds to polar unsaturated bonds. Chem. Rev. 115, 3468–3517 (2015).
Wu, L. et al. Palladium-catalyzed carbonylative transformation of C(sp3)–X bonds. ACS Catal. 4, 2977–2989 (2014).
Liu, B., Hu, F. & Shi, B.-F. Recent advances on ester synthesis via transition-metal catalyzed C–H functionalization. ACS Catal. 5, 1863–1881 (2015).
Wu, X.-F., Neumann, H. & Beller, M. Palladium-catalyzed oxidative carbonylation reactions. ChemSusChem 6, 229–241 (2013).
Liu, Q., Zhang, H. & Lei, A. Oxidative carbonylation reactions: organometallic compounds (R–M) or hydrocarbons (R–H) as nucleophiles. Angew. Chem. Int. Ed. 50, 10788–10799 (2011).
Yoo, E. J., Masa, M. & Yu, J.-Q. Pd(II)-catalyzed carbonylation of C(sp3)-H bonds: a new entry to 1,4-dicarbonyl compounds. J. Am. Chem. Soc. 132, 17378–17380 (2010).
McNally, A., Haffemayer, B., Collins, B. S. L. & Gaunt, M. J. Palladium-catalyzed C–H activation of aliphatic amines to give strained nitrogen heterocycles. Nature 510, 129–133 (2014).
Li, S., Chen, G., Feng, C.-G., Gong, W. & Yu, J.-Q. Ligand-enabled γ-C-H olefination and carbonylation: construction of β-quaternary carbon centers. J. Am. Chem. Soc. 136, 5267–5270 (2014).
Wang, P.-L. et al. Pd-catalyzed C(sp3)-H carbonylation of alkylamines: a powerful route to γ-lactams and γ-amino acids. Org. Lett. 17, 3698–3701 (2015).
Wang, C. et al. Oxalyl amide assisted-palladium-catalyzed synthesis of pyrrolidones via carbonylation of γ-C(sp3)-H bonds of aliphatic amine substrates. Chem. Sci. 6, 4610–4615 (2015).
Calleja, J. et al. A steric tethering approach enables palladium-catalyzed C-H activation of primary amino alcohols. Nat. Chem. 7, 1009–1016 (2015).
Xie, P. et al. Palladium-catalyzed oxidative carbonylation of benzylic C-H bonds via nondirected C(sp3)-H activation. J. Am. Chem. Soc. 134, 9902–9905 (2012).
Xie, P., Xia, C. & Huang, H. Palladium-catalyzed oxidative aminocarbonylation: a new entry to amides via C–H activation. Org. Lett. 15, 3370–3373 (2013).
Li, Z.-Y. & Wang, G.-W. Palladium-catalyzed decarboxylative ortho-ethoxycarbonylation of O-methyl ketoximes and 2-arylpyridines with potassium oxalate monoester. Org. Lett. 17, 4866–4869 (2015).
Chen, J., Feng, J.-B., Natte, K. & Wu, X.-F. Palladium-catalyzed carbonylative cyclization of arenes by C-H bond activation with DMF as the carbonyl source. Chem. Eur. J 21, 16370–16373 (2015).
Wu, J., Lan, J., Guo, S. & You, J. Pd-catalyzed C–H carbonylation of (hetero)arenes with formates and intramolecular dehydrogenative coupling: a shortcut to indolo[3,2,c]coumarins. Org. Lett. 16, 5862–5865 (2014).
Huang, Y., Li, G., Huang, J. & You, J. Palladium-catalyzed direct ortho-C-H ethoxycarboxylation of anilides at room temperature. Org. Chem. Front. 1, 347–350 (2014).
Yu, W.-Y., Sit, W. N., Lai, K.-M., Zhou, Z. & Chan, A. S. C. Palladium-catalyzed oxidative ethoxycarbonylation of aromatic C–H bond with diethyl azodicarboxylate. J. Am. Chem. Soc. 130, 3304–3306 (2008).
Zhou, W., Li, P., Zhang, Y. & Wang, L. Palladium-catalyzed directed alkoxycarbonylation of aromatic C-H bonds via selective C–C cleavage of α-keto esters. Adv. Synth. Catal. 355, 2343–2352 (2013).
Wang, S. et al. Efficient synthesis of anthranilic esters via Pd-catalyzed dehydrogenative/decarbonylative coupling of anilides and glyoxylates. Chem. Commun. 48, 9924–9926 (2012).
Peng, X., Zhu, Y., Ramirez, T. A., Zhao, B. & Shi, Y. New reactivity of oxaziridine: Pd(II)-catalyzed aromatic C–H ethoxycarbonylation via C–C bond cleavage. Org. Lett. 13, 5244–5247 (2011).
Kochi, T. et al. Ruthenium-catalyzed amino- and alkoxycarbonylations with carbamoyl chlorides and alkyl chloroformates via aromatic C–H bond cleavage. J. Am. Chem. Soc. 131, 2792–2793 (2009).
Chen, F.-J. et al. Pd(II)-catalyzed alkoxylation of unactivated C(sp3)-H and C(sp2)-H bonds using a removable directing group: efficient synthesis of alkyl ethers. Chem. Sci. 4, 4187–4192 (2013).
Zhang, Q. et al. Stereoselective synthesis of chiral α-amino-β-lactams through palladium(II)-catalyzed sequential monoarylation/amidation of C(sp3)-H bonds. Angew. Chem. Int. Ed. 52, 13588–13592 (2013).
Zhang, Q., Yin, X.-S., Chen, K., Zhang, S.-Q. & Shi, B.-F. Stereoselective synthesis of chiral β-fluoro α-amino acids via Pd(II)-catalyzed fluorination of unactivated methylene C(sp3)-H bonds: scope and mechanistic studies. J. Am. Chem. Soc. 137, 8219–8226 (2015).
Reddy, B. V. S., Reddy, L. R. & Corey, E. J. Novel acetoxylation and C–C coupling reactions at unactivated positions in α-amino acid derivatives. Org. Lett. 8, 3391–3394 (2006).
Tran, L. D. & Daugulis, O. Nonnatural amino acid synthesis by using carbon-hydrogen bond functionalization methodology. Angew. Chem. Int. Ed. 51, 5188–5191 (2012).
Feng, Y. & Chen, G. Total synthesis of celogentin C via stereoselective C–H activation. Angew. Chem. Int. Ed. 49, 958–961 (2010).
He, G., Wang, B., Nack, W. A. & Chen, G. Synthesis and transformations of α-amino acids via palladium-catalyzed auxiliary-directed sp3 C–H functionalization. Acc. Chem. Res. 49, 635–645 (2016).
Zaitsev, V. G., Shabashov, D. & Daugulis, O. Highly regioselective arylation of sp3 C–H bonds catalysed by palladium acetate. J. Am. Chem. Soc. 127, 13154–13155 (2015).
Shabashov, D. & Daugulis, O. Auxiliary-assisted palladium-catalyzed arylation and alkylation of sp2 and sp3 carbon-hydrogen bonds. J. Am. Chem. Soc. 132, 3965–3972 (2010).
Daugulis, O., Roane, J. & Tran, L. D. Bidentate, monoanionic auxiliary-directed functionalization of carbon-hydrogen bonds. Acc. Chem. Res. 48, 1053–1064 (2015).
Rit, R. K., Yadav, M. R., Ghosh, K. & Sahoo, A. K. Reusable directing groups [8-aminoquinoline, picolinamide, sulfoximine] in C(sp3)-H bond activation: present and future. Tetrahedron 71, 4450–4459 (2015).
Rouquet, G. & Chatani, N. Catalytic functionalization of C(sp2)-H and C(sp3)-H bonds by using bidentate directing groups. Angew. Chem. Int. Ed. 52, 11726–11743 (2013).
Chen, K. & Shi, B.-F. Sulfonamide-promoted palladium(II)-catalyzed alkylation of unactivated methylene C(sp3)-H bonds with alkyl iodides. Angew. Chem. Int. Ed. 53, 11950–11954 (2014).
Chen, K., Hu, F., Zhang, S.-Q. & Shi, B.-F. Pd(II)-catalyzed alkylation of unactivated C(sp3)-H bonds: efficient synthesis of optically active unnatural α-amino acids. Chem. Sci. 4, 3906–3911 (2013).
Barnard, C. F. J. Palladium-catalyzed carbonylation–a reaction come of age. Organometallics 27, 5402–5422 (2008).
Brennfuhrer, A., Neumann, H. & Beller, M. Palladium-catalyzed carbonylation reactions of aryl halides and related compounds. Angew. Chem. Int. Ed. 48, 4114–4133 (2009).
Topczewski, J. & Sanford, M. S. Carbon-hydrogen (C–H) bond activation at PdIV: a Frontier in C–H functionalization catalysis. Chem. Sci 6, 70–76 (2014).
Hickman, A. J. & Sanford, M. S. High-valent organometallic copper and palladium in catalysis. Nature 484, 177–185 (2012).
Xu, L.-M., Li, B.-J., Yang, Z. & Shi, Z.-J. Organopalladium(IV) chemistry. Chem. Soc. Rev. 39, 712–733 (2010).
Muñiz, K. High-oxidation-state palladium catalysis: new reactivity for organic synthesis. Angew. Chem. Int. Ed. 48, 9412–9423 (2009).
Powers, D. C. & Ritter, T. Palladium(III) in synthesis and catalysis. Top. Organomet. Chem 35, 129–156 (2011).
Wencel-Delord, J. & Glorius, F. C-H bond activation enables the rapid construction and late-stage diversification of functional molecules. Nat. Chem. 5, 369–375 (2013).
Dailler, D., Danoun, G. & Baudoin, O. A general and scalable synthesis of aeruginosin marine natural products based on two strategic C(sp3)-H activation reactions. Angew. Chem. Int. Ed. 54, 4919–4922 (2015).
Dailler, D., Danoun, G., Ourri, B. & Baudoin, O. Divergent synthesis of aeruginosins based on a C(sp3)-H activation strategy. Chem. Eur. J 21, 9370–9379 (2015).
Tremont, S. J. Ortho-alkylation of acetanilides using alkyl halides and palladium acetate. J. Am. Chem. Soc. 106, 5759–5760 (1984).
Daugulis, O. & Zaitsev, V. G. Anilide ortho-arylation by using C–H activation methodology. Angew. Chem. Int. Ed. 44, 4046–4048 (2005).
Zhu, C., Zhang, Y., Kan, J., Zhao, H. & Su, W. Ambient-temperature ortho C–H arylation of benzoic acids with aryl iodides with ligand-supported palladium catalyst. Org. Lett. 17, 3418–3421 (2015).
Kozitsyna, N. Y. et al. Novel heterometallic palladium–silver complex. Inorg. Chim. Acta 370, 382–387 (2011).
Acknowledgements
Financial support from the National Basic Research Program of China (2015CB856600) and the NSFC (21572201, 21422206, 21272206) is gratefully acknowledged. We thank Prof. Xueqian Kong from Zhejiang University, China for help with the use of facilities in his lab.
Author information
Authors and Affiliations
Contributions
G.L. and B.-F.S. conceived and designed the study. G.L. principally performed the experiments. X.-S.Y., K.C., Q.Z. and S.-Q.Z. helped to conduct some experiments and collect data. B-F.S. provided overall supervision and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
B.-F.S., G.L. and X.-S.Y. are the co-inventors on a patent application: ‘A highly efficient method to the synthesis of aspartate derivatives’ CN application number 201610147639.1. The remaining authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-67, Supplementary Tables 1-12, Supplementary Methods and Supplementary References (PDF 13707 kb)
Supplementary Data 1
X-ray CIF file for complex III (CCDC 1446624) (CIF 25 kb)
Supplementary Data 2
X-ray CIF file for compound 3a (CCDC 1487147) (CIF 22 kb)
Supplementary Data 3
X-ray CIF file for compound 4b (CCDC 1486639) (CIF 21 kb)
Supplementary Data 4
X-ray CIF file for compound 3d (CCDC 1486599) (CIF 27 kb)
Supplementary Data 5
X-ray CIF file for compound 3k (CCDC 1446623) (CIF 22 kb)
Supplementary Data 6
X-ray CIF file for compound 6a (CCDC 1475241) (CIF 19 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Liao, G., Yin, XS., Chen, K. et al. Stereoselective alkoxycarbonylation of unactivated C(sp3)–H bonds with alkyl chloroformates via Pd(II)/Pd(IV) catalysis. Nat Commun 7, 12901 (2016). https://doi.org/10.1038/ncomms12901
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms12901
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.