Abstract
Among the emerging renewable and green energy sources, biohydrogen stands out as an appealing choice. Hydrogen can be produced by certain groups of microorganisms that possess functional nitrogenase and/or bidirectional hydrogenases. In particular, the potential of photobiological hydrogen production by oxygenic photosynthetic microbes has attracted significant interest. However, nitrogenase and hydrogenase are generally oxygen sensitive, and require protective mechanisms to function in an aerobic extracellular environment. Here, we describe Cyanothece sp. ATCC 51142, a unicellular, diazotrophic cyanobacterium with the capacity to generate high levels of hydrogen under aerobic conditions. Wild-type Cyanothece 51142 can produce hydrogen at rates as high as 465 μmol per mg of chlorophyll per hour in the presence of glycerol. Hydrogen production in this strain is mediated by an efficient nitrogenase system, which can be manipulated to convert solar energy into hydrogen at rates that are several fold higher, compared with any previously described wild-type hydrogen-producing photosynthetic microbe.
Similar content being viewed by others
Introduction
Microbial H2 production relies on either photosynthetic or fermentative processes. Economic feasibility studies suggest that a direct and efficient conversion of solar energy to H2 in a carbon-neutral way is necessary for the process to be commercially viable1,2. Several photosynthetic microalgal and bacterial species possessing nitrogenase and/or hydrogenase enzymes are being studied as prospective model organisms for photobiological H2 production3,4,5. Outstanding among them are Rhodopseudomonas palustris, a purple photosynthetic, nitrogen-fixing bacterium; Chlamydomonas reinhardtii, a green eukaryotic alga; as well as Anabaena and Synechocystis, members of the cyanobacterial group. High specific rates of nitrogenase-mediated H2 production have been reported for R. palustris6. However, R. palustris performs anoxygenic photosynthesis, thereby requiring an anaerobic environment for photobiological H2 production7,8,9. Anabaena, a filamentous diazotrophic cyanobacterial strain, produces H2 in heterocysts, specialized N2-fixing cells, which maintain a microaerobic environment to protect the oxygen-sensitive nitrogenase enzyme. However, the low frequency of heterocysts in a filament (about one in ten cells normally differentiates into heterocysts10) consequently results in modest yields of net H2 production. In contrast, H2 production in C. reinhardtii and Synechocystis sp. PCC 6803 is mediated by hydrogenase enzymes, and H2 production can be achieved in both of these organisms only under strictly anaerobic conditions11,12,13. Although these strains have long been used as model organisms to study biohydrogen production, the importance of selecting additional novel and native strains with diverse energy conversion systems that might have evolved as a consequence of specific ecological pressure has often been emphasized1,14. A recent effort in this direction has identified ten native N2-fixing, heterocystous cyanobacterial strains that exhibit higher rates of H2 production compared with some of the previously studied mutant strains15.
In the present report, we describe Cyanothece sp. ATCC 51142, a cyanobacterial strain with the ability to produce remarkably high amounts of H2 under aerobic conditions. Cyanothece strains thrive in marine environments limited in dissolved inorganic bioavailable nitrogenous compounds, and have been recognized for their role in maintaining the marine nitrogen cycle16,17,18. Cyanothece 51142 can derive the majority of its nutritional requirements from sunlight, atmospheric carbon dioxide and nitrogen gases (Fig. 1). During the day, it performs photosynthesis and fixes carbon, which is stored as large reserves of glycogen19. At the onset of the dark period, high rates of respiration rapidly create a suboxic intracellular environment. This, in turn, facilitates oxygen-sensitive and energy-intensive processes such as N2 fixation and H2 production to occur at night at the expense of the accumulated glycogen. The orchestrated diurnal cycling patterns of the central metabolic processes in this organism have recently been described using a global transcriptomic approach20. These unique attributes of Cyanothece 51142, which make it an ideal organism for H2 production, are possibly the remnants of the metabolic and regulatory processes that aided in the acclimatization of ancient cyanobacteria during their transition from an anaerobic to an aerobic environment. Retention of ancient metabolic traits that originated in the Archaean oceans has been reported in other cyanobacterial strains21.
Results
A two-stage system for photobiological H2 production
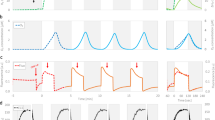
We developed a two-stage H2 production system in Cyanothece 51142 on the basis of our previous knowledge of the diurnal rhythms in this unicellular cyanobacterium22,23. The two stages were comprised of a growth phase during which cells were allowed to grow aerobically under 12 h light/12 h dark cycles, followed by an incubation phase during which cells sampled at the end of the 12 h light period were incubated in airtight vials under continuous illumination for 12 h. During this latter light-incubation period, the physiological activities of the cells were in step with the subjective dark condition, thereby facilitating N2 fixation and H2 production. At the completion of the incubation phase, the headspace of the vials (which contained 100% air at the beginning of incubation) was analysed for H2 accumulation. We determined that photoautotrophically grown Cyanothece 51142 exhibited high specific rates of H2 production (>150 μmol of H2 per mg of chlorophyll per hour (Chl.h) or 2.34 μmol of H2 per mg of dry weight per hour) under aerobic incubation conditions (Fig. 2a). This is striking, as most unicellular photosynthetic microbial strains require a complete anaerobic environment for H2 production24 (Table 1).
(a) Specific rates of H2 production by Cyanothece 51142 grown under aerobic, photoautotrophic conditions using ambient (0.03%, v/v) or high (8%, v/v) concentrations of CO2, and under photomixotrophic growth conditions with glycerol (50 mM). Cultures were incubated under aerobic conditions for H2 production. Each column represents an average of measurements from at least three biological replicates. Error bars indicate s.d. values from the average. (b) Kinetics of aerobic photobiological H2 production in a batch culture of Cyanothece 51142. H2 production (blue) could be observed for more than 60 h in vials containing cultures under aerobic incubation conditions in the presence of light. The respiratory activities of the cells resulted in a gradual decrease in O2 concentration in the headspace of the vials (red). Three biological replicates were used for the measurements. Error bars indicate s.d. values from the average. (c) Comparison of cumulative H2 accumulation in vials with cells under light and dark incubation conditions. A volume of 916 ml of H2 per litre of culture accumulated in the vials when incubated in the light compared with 7 ml of H2 per litre of culture accumulation in the dark. Each column represents an average of measurements from at least three biological replicates. Error bars indicate s.d. values from the average. (d) Dissolved O2 levels in a batch culture of Cyanothece 51142 cells under H2-producing conditions. The circadian rhythms of respiratory and photosynthetic activities were reflected in the dissolved O2 concentration of the culture. The culture was transferred to incubation vials at the beginning of the dark period when the dissolved O2 level was about 50% of the maximum (assuming 100% equals 230 μM dissolved O2 at 30 °C). The O2 concentration reduced starting from the middle of the subjective light period (blue line, grey bar), was at its minimum at the middle of the subjective dark period (red line, white bar), after which an increase was observed because of photosynthetic oxygen evolution. The horizontal bars below the x axis denote subjective day (open) and night (filled) periods. Three independent biological replicates were used for the measurements. Error bars indicate s.d. values from the average.
The rates of H2 production in Cyanothece 51142 could be greatly enhanced when cells were grown in the presence of additional carbon sources, as observed in cultures supplemented with high concentrations of CO2, or glycerol (Fig. 2a). Cells grown under CO2-enriched air and incubated under aerobic conditions could produce 230 μmol of H2 per mg of Chl.h (Fig. 2a). In fact, a batch culture of Cyanothece 51142 cells supplemented with 50 mM glycerol could produce more than 900 ml of H2 per litre of culture over a period of 2 days (Fig. 2b,c) when incubated in constant light under aerobic conditions (Fig. 2b). Notably, the kinetics of H2 production under these conditions revealed high rates even early in the incubation phase when the O2 concentration in the headspace of the vials was equivalent to that in air (Fig. 2b), indicating that an intracellular anoxic environment facilitates nitrogenase activity during the subjective dark period. The O2 level in the headspace diminished with time (Fig. 2b), indicating continued respiratory activities in the cells. Moreover, as shown in the next subsection, transcripts of coxA, the gene encoding subunit A of the cytochrome c oxidase enzyme involved in respiration, were also detected under these conditions. This respiration-induced microaerobic environment was also reflected in the dissolved O2 levels in the culture in the incubation vials (Fig. 2d). A rapid decline in the dissolved O2 concentration was observed at the beginning of the incubation phase, followed by periodic oscillations in O2 levels, which corresponded to the respiratory and photosynthetic activity of the cells in the subjective dark and light periods, respectively. In comparison with light incubation, incubation under dark conditions reduced the level of H2 production by more than 100-fold, indicating that H2 production in this system is driven by light energy, and the high rates observed cannot be achieved by dark fermentation of glycerol alone (Fig. 2c).
Additional carbon sources lead to higher glycogen content
We assessed the influence of external carbon sources on the cellular energy reserves, a critical determinant of nitrogenase activity and H2 production. The glycogen levels of the cells were compared between pre- (I0) and post-incubation (I12) samples collected from cultures grown under ambient CO2 (0.03%), under 8% CO2-enriched air, and with 50 mM glycerol. Either high CO2 or glycerol enhanced the glycogen reserves of the cells (Fig. 3). In ambient and high CO2-treated cells, the glycogen level diminished at the end of the incubation period, indicating that the endogenous carbon source was being used for H2 production (Fig. 2a). In contrast, cells grown in the presence of glycerol showed higher levels of glycogen at the end of the 12 h-light incubation phase (I12), implying direct utilization of glycerol as a source of ATP and reductants by the nitrogenase enzyme under this photomixotrophic condition. This is supported by the observation that expression of glycogen phosphorylase (glgP), a gene involved in glycogen degradation, was also downregulated in glycerol-supplemented cells (Fig. 4b). In this context, we have recently shown that Cyanothece 51142 uses glycerol as the sole carbon source when cells are grown under photomixotrophic conditions in the presence of glycerol25.
I0 (red) and I12 (blue) indicate the beginning and end of a 12 h-light incubation period for H2 production, respectively. Differences in glycogen level between the two time points correspond to the amount of glycogen used for N2 fixation/H2 production. Each column represents an average of measurements from at least three biological replicates. Error bars indicate s.d. values from the average.
(a) Comparisons of the expression of genes involved in H2 metabolism under N2-fixing (−NO3) and nitrogen-sufficient (+NO3) conditions from cultures grown under 12 h light/dark cycles. nifH transcripts could only be observed under N2-fixing condition, whereas hoxH and hupS transcripts were present under both conditions. coxA transcripts were predominant during the dark cycle both under N2-sufficient and N2-deficient conditions. Transcripts for glgP were abundant during the dark cycle under both conditions. 16S rRNA was used as the loading control. Cells were collected at 4 h intervals, between D1 (1 h after the onset of the dark cycle) and L9 (9 h into the light cycle). (b) Reverse transcription PCR analysis of the enzyme systems involved in H2 metabolism in Cyanothece 51142. Samples were collected from the incubation bottles (continuous light, aerobic) at the beginning (I0) and end of the incubation phase (I12). Strong nifH expression could be observed at I0, in the control (grown in ambient CO2) as well as in cultures supplemented with high concentrations of CO2 or glycerol. nifH transcripts were also present in cells incubated under light during H2 production (I12). Expression of glgP was downregulated in cultures supplemented with glycerol, indicating that glycerol, and not glycogen, is used directly as a carbon source under these mixotrophic conditions. Interestingly, hoxH expression was also significantly downregulated in the presence of glycerol. Expression of hupS was lower under light. The presence of coxA transcripts suggested respiratory activities in the vials under all conditions. 16S rRNA was used as the loading control. –RTase denotes controls to demonstrate absence of DNA contamination in the samples. (c) Addition of NaNO3 to the medium of an N2-fixing culture resulted in cessation of H2 production (blue) within 12 h and greatly reduced nitrogen fixation (green). Nitrogenase activity is expressed in terms of ethylene production by acetylene reduction. Each column represents an average of measurements from at least three biological replicates. Error bars indicate s.d. values from the average.
Hydrogen production is mediated by the nitrogenase enzyme
Genome analysis of Cyanothece 51142 revealed the presence of both the nitrogenase (Nif) and bidirectional hydrogenase (Hox) enzyme systems. Analysis at the transcriptional level revealed that hox genes were expressed under both nitrogen-fixing (−NO3) and nitrogen-sufficient (+NO3) conditions (Fig. 4a), and higher transcript abundance could be associated with the dark period under both conditions. In contrast, nif genes were expressed only under nitrogen-fixing conditions, and, in the absence of any additional carbon source, the nif transcripts were strictly associated with the dark period (Fig. 4a). High levels of H2 production in Cyanothece 51142 were detected exclusively under diazotrophic conditions, and addition of combined nitrogen to the growth media of nitrogen-fixing cultures resulted in an immediate reduction in the rates of nitrogen fixation and simultaneous cessation of H2 production (Fig. 4c). These results show that the high rates of H2 production observed in Cyanothece 51142 were primarily mediated by the nitrogenase enzyme system.
Nitrogenase can be optimized to enhance hydrogen yield
An important feature of the nitrogenase enzyme system is the potential to channel all available electrons towards H2 production in the absence of molecular nitrogen26,27. The nitrogenase reaction is also resistant to feedback inhibition from accumulated H2 (ref. 28). When Cyanothece 51142 cells grown under photoautotrophic conditions were incubated under an argon atmosphere (absence of molecular nitrogen), a 2- to 3-fold increase (>370 μmol of H2 per mg of Chl.h or 5.738 μmol of H2 per mg of dry weight per hour) in the yield of H2 was achieved (Fig. 5, Tables 1 and 2). In addition, production rates of up to 467 μmol of H2 per mg of Chl.h were achieved from cells grown in glycerol-supplemented media subsequently incubated under argon environment (Fig. 5). These rates are at least an order of magnitude higher compared with any other hydrogen-producing photosynthetic microbial wild-type strain studied to date (Tables 1 and 2).
Higher specific rates of H2 production are achieved in the absence of molecular N2 in the headspace of the incubation bottles. Each column represents an average of measurements from at least three biological replicates in air (blue) and argon (red) atmospheres. Error bars indicate s.d. values from the average.
Discussion
This work describes the use of the wild-type strain of the unicellular cyanobacterium Cyanothece 51142 for highly efficient photobiological H2 production under natural aerobic conditions. In general, the oxygen sensitivity of the enzymes involved in biological H2 production makes the use of oxygenic photosynthetic organisms as a platform for H2 production an extremely challenging task. Thus, until now, photobiological H2 production studies have largely relied on artificial interventions, which help to create an anaerobic environment. It has been recognized that the use of oxygenic photosynthetic microbes that can produce H2 under aerobic conditions would be an important step forward for biological H2 production29. Our study has identified Cyanothece 51142 as one such organism that has developed an effective strategy to make the best use of a diurnal cycle—synthesizing energy-rich storage compounds during the day and using it for nitrogen fixation at night when oxygen-consuming processes render the interior of the cell anaerobic (or suboxic), while the extracellular environment continues to be oxygen rich. This trait of Cyanothece 51142 formed the basis for the physiological perturbations that were designed for our two-stage aerobic H2 production process.
Nitrogen fixation is an energy-intensive process, requiring 16 molecules of ATP for every molecule of nitrogen fixed and H2 produced19 (Fig. 1). However, the process is of paramount importance to diazotrophic species inhabiting ecological niches (such as deep oceans) with very low levels of nitrogenous nutrients. Consequently, photoautotrophic unicellular strains such as Cyanothece 51142 are expected to have evolved effective strategies for collecting and storing solar energy, which can be used at night when the energy demands are high. Our study shows that Cyanothece 51142 not only develops an intracellular environment conducive for the function of the nitrogenase enzyme but also generates an adequate supply of ATP for this high-energy-requiring process. In addition, our results reveal high specific activity of the nitrogenase enzyme in this strain, a finding consistent with earlier reports that showed higher rates of nitrogen fixation in marine unicellular diazotrophs such as Cyanothece compared with some filamentous strains16,30. A unicellular, marine, diazotrophic Synechococcus strain (Synechococcus Miami BG 43511; later classified as Cyanothece Miami BG 4351116) was also shown to exhibit high rates of nitrogenase activity and hydrogen production31. Unfortunately, this strain has not been readily accessible to the general research community. Notably, not all unicellular cyanobacterial strains are endowed with these traits, as is evident from studies on Gloeothece, a unicellular freshwater diazotroph that possesses a nitrogenase enzyme with relatively low specific activity and does not have any appreciable nitrogenase-mediated H2 production capacity32.
Diazotrophic cyanobacteria have developed various strategies to protect their nitrogenase enzyme from the oxygen-rich environment they inhabit. However, unlike Cyanothece 51142, most diazotrophic strains are unable to exhibit nitrogenase-mediated H2 production under aerobic conditions. In filamentous, heterocyst-forming diazotrophic strains, this is largely ascribed to the activities of an uptake hydrogenase enzyme system that is functionally closely associated with nitrogenase and oxidizes the H2 produced3,33,34,35. It has been shown that wild-type Anabaena variabilis cells can generate H2 only under an argon atmosphere, whereas its uptake hydrogenase mutants PK84 and AVM13 can produce H2 aerobically27,36,37,38. A recent study also demonstrated H2 production (∼25 μmol per mg of Chl.h) from the vegetative cells of wild-type Anabaena variabilis under nitrogen atmosphere when strict anaerobic conditions are maintained39. The genome sequence of Cyanothece 51142 shows the presence of hup genes for an uptake hydrogenase. The transcripts for one of these genes, hupS, were also detected under H2-producing conditions (Fig. 4b). Interestingly, the hupS transcripts in Cyanothece 51142 were present under both nitrogen-sufficient and nitrogen-fixing conditions (Fig. 4a), indicating that its expression is independent of nif. The presence of hupS transcripts and the concurrent accumulation of H2 at high rates under aerobic incubation conditions are suggestive of a weak uptake hydrogenase activity in Cyanothece 51142. Such a premise is also supported by the observation that a few wild-type Anabaena strains that possess uptake hydrogenases with very low specific activities can also exhibit aerobic H2 production24,40,41.
The ability to use high concentrations of CO2 or glycerol for enhanced H2 production is an added advantage, as both of these carbon sources are abundantly available as industrial waste products, making biohydrogen production by Cyanothece 51142 an attractive option. High CO2 and glycerol provide an additional carbon source and the availability of excess carbon functions as a signal for enhanced nitrogenase activity to meet the increase in nitrogen demand in these cyanobacterial cells. The rise in the glycogen level of cells at the end of the incubation phase in glycerol-supplemented cultures could be a result of cellular activities geared towards building energy reservoirs when an external energy source is readily available21.
Decades of research have unveiled various principles underlying biological H2 production. However, achieving significant increases in yield has been a major challenge. Genetic modifications of H2-yielding pathways have resulted in improvements in production rates compared with the corresponding wild-type strains9,42,43. However, as the H2 production rates in these wild-type strains are rather modest, even a 20-fold increase in yield in mutant strains is not sufficient to attain a high production level. Therefore, our identification of a cyanobacterial strain exhibiting high rates of H2 production under ambient aerobic conditions offers new possibilities in photobiological hydrogen production research. Recent studies have revealed the metabolic flexibility of this cyanobacterium25, and demonstrated that its robust circadian rhythm allows N2 fixation and H2 production to occur at reasonably high rates even when grown under continuous light44. Previous studies have shown the robustness of other cyanobacterial systems for H2 production over a prolonged period of time38, demonstrating the possibility of using high-H2-yielding cyanobacterial strains for large-scale production. A systems level understanding of this biological phenomenon in Cyanothece 51142 will unravel previously unknown cellular factors and regulatory mechanisms that influence the process so that they can be favourably altered to produce even higher levels of H2 as an energy carrier.
Methods
Growth conditions
For H2 measurement, Cyanothece 51142 cells were grown in shaking flasks in ASP2 medium45 without supplemented NaNO3 at 30 °C under 12 h light/12 h dark cycles and 100 μmol photons per m2 s−1 of white light. Cultures were inoculated with 0.25 volumes of cultures grown in ASP2 medium without NaNO3 under continuous light (50 μmol photon per m2 s−1 white light), which in turn were inoculated with 0.1 volumes of cultures grown in ASP2 with NaNO3 under similar conditions. For photomixotrophic growth, cultures were supplemented with 50 mM glycerol. For growth under high-CO2 conditions, the cultures were aerated with 8% CO2-enriched air at a flow rate of 100 ml min−1.
H2 production and nitrogenase activity measurement
A volume of 20 ml of culture was transferred at the beginning of the dark period to air-tight glass vials (36 ml) and incubated in air under a light intensity of 100 μmol photon per m2 s−1 for 12 h. The chlorophyll content of cultures grown without an external carbon source ranged between 0.5 and 2 μg ml−1, whereas cultures supplemented with glycerol had higher chlorophyll concentration (2–5 μg ml−1). Dry weight of photoautotrophically grown culture ranged between 90 and 98 μg ml−1. For anaerobic incubation, the glass vials were flushed with argon for 15–30 min. For the batch culture experiment, 25 ml of dense (8–12 μg chlorophyll per ml culture) glycerol-supplemented culture was incubated in 145 ml vials under constant illumination. H2 that accumulated in the headspace of sealed culture vials was withdrawn with an air-tight syringe and quantified using an Agilent 6890N Gas Chromatograph (Agilent) equipped with a Molsieve 5A 60/80 column (Molsieve; inner dimensions 6′×1/8″) and a thermal conductivity detector. Injection port, oven and detector temperatures were 100, 50 and 100 °C, respectively. Argon, the carrier gas, was supplied at a flow rate of 65 ml min−1. The volume of gas expressed in the results section was under standard conditions (assuming 1 ml H2=44.6 μmol).
Nitrogenase activity of the H2-producing cultures was determined using an acetylene reduction assay46 and expressed in terms of the ethylene produced. Cells were incubated in sealed glass vials in light at 30 °C under a 5% acetylene atmosphere with or without flushing with argon. Gas samples were withdrawn, and ethylene production was measured using an Agilent 6890N Gas Chromatograph (Agilent) equipped with a Poropak N column (inner dimensions 5′×I/8″) and a flame ionization detector using argon as the carrier gas (flow rate of 65 ml min−1), according to the manufacturer's instructions. The temperature of the injector, detector and oven were 150, 200 and 100 °C, respectively.
Total chlorophyll a was extracted by methanol and quantified spectrophotometrically using an Olis DW2000 spectrophotometer (On-Line Instrument Systems). Protein concentrations were determined using a bicinchoninic acid assay (Pierce) according to the manufacturer's instructions.
Oxygen measurements
O2 concentrations in the headspace of the incubation vials were determined using an Agilent 6890N Gas Chromatograph (Agilent) equipped with a Molsieve 5A 60/80 column (Molsieve; inner dimensions 6′×1/8″) and a thermal conductivity detector using the same settings as described above for the H2 assay. The measurements were taken under standard conditions. Dissolved O2 concentration in the incubation vials was measured using a Clark-type electrode. Calculations were based on the fact that air-saturated water contains 230 μM of O2 at 30 °C.
Determination of glycogen content
Samples were collected for the glycogen assay at the beginning and end of the 12 h-light incubation phase of H2 production. The cellular glycogen content was measured using a glucose hexokinase assay (Sigma) with glycogen from bovine liver Type IX (Sigma) as standard. After methanol extraction of chlorophyll, the cell pellets were washed twice with 100% ethanol. To remove free glucose, 40% KOH was added and the samples were incubated for 1 h at 95 °C. Glycogen was precipitated overnight at −20 °C with 2 volumes of 100% ethanol. The samples were centrifuged for 1 h at 4 °C and 2 N HCl was added before incubation at 95 °C for 30 min. The same volume of 2 N NaOH and 0.5 volumes of 1 M phosphate buffer, pH 7, were added before dilution with 1 volume of distilled water. For the enzyme assay, 75 μl of sample solution was mixed with 200 μl of enzyme solution in a microtitre plate (Costar, ultraviolet light proof). After 15 min incubation at ambient temperature, NADPH was measured at 340 nm on a μQuant plate reader (Bio-Tek Instruments).
Semiquantitative reverse transcription (RT–PCR)
Semiquantitative RT–PCR analyses were performed on RNA samples isolated from cultures grown under nitrogen-fixing (−NO3) conditions, with and without supplemented glycerol; from cultures grown under aeration with 8% CO2-enriched air; and under non-nitrogen-fixing conditions (+NO3). For the time-course experiment, samples were collected every 4 h for 24 h, starting with 1 h into the dark period (D1). In total, six samples were collected. For RT–PCR analysis under H2-producing conditions, culture samples were assayed at the end of the light period at time point I0 and from the assay bottles at the end of incubation at time point I12. RNA was isolated and quantified essentially as described in ref. 14. A volume of 700 ng of DNase (Promega)-treated total RNA samples was used for reverse transcription with the Superscript II Reverse Transcriptase and random primers (Invitrogen) according to the manufacturer's instructions. The absence of DNA contamination was tested for each RNA sample (Fig. 4b). PCR was carried out at 94 °C for 4 min, followed by 94 °C for 30 s, 58 °C for 20 s, 72 °C for 20 s and a final extension time of 4 min at 72 °C. A total of 25 cycles for the nifH, hupS, glgP and coxA genes and 26 cycles for the hoxH gene were used. The following primers were used: nifH F: 5′-ACCATTGCTGCGTTAGCTGAAAC-3′, R: 5′-TAATACCACGACCCGCACATCCA-3′; coxA F: 5′-TGATATGGCCTTTCCCACCCTCA-3′, R: 5′-AGAGAACTAAAGCGGCAGCGAGA-3′; hupS F: 5′-ATAGCTGGTTTCGTTGTCGCTGT-3′, R: 5′-CGAAGTCTTGGGTGGTTGCTTTG-3′; hoxH F: 5′-TGGAGAAGACGGACTTTGGGAAC-3′, R: 5′-AAAGAAGAGGTCGCTACACCACC-3′; glgP F: 5′-TCGGCTGAATTCCTTATGGGTCG-3′, R: 5′-CAGGAATTTCCACTTGCCAACCG-3′; 16S rRNA F: 5′-AGAGGATGAGCAGCCACACT-3′, R: 5′-TAATTCCGGATAACGCTTGC-3′ (F: forward, R: reverse).
Additional information
How to cite this article: Bandyopadhyay, A. et al. High rates of photobiological H2 production by a cyanobacterium under aerobic conditions. Nat. Commun. 1:139 doi: 10.1038/ncomms1139 (2010).
References
Carrieri, D., Kolling, D., Ananyev, G. & Dismukes, G. C. Prospecting for biohydrogen fuel. Indstrl. Biotechnol. 2, 133–137 (2006).
Das, D. & Veziroglu, T. N. Advances in biological hydrogen production processes. Int. J. Hydrogen Energy 33, 6046–6057 (2008).
Tamagnini, P. et al. Hydrogenases and hydrogen metabolism of cyanobacteria. Microbiol. Mol. Biol. Rev. 66, 1–20 (2002).
Ghirardi, M. L. et al. Hydrogenases and hydrogen photoproduction in oxygenic photosynthetic organisms. Annu. Rev. Plant Biol. 58, 71–91 (2007).
Tamagnini, P. et al. Cyanobacterial hydrogenases: diversity, regulation and applications. FEMS Microbiol. Rev. 31, 692–720 (2007).
Melnicki, M. R., Bianchi, L., De Philippis, R. & Melis, A. Hydrogen production during stationary phase in purple photosynthetic bacteria. Int. J. Hydrogen Energy 33, 6525–6534 (2008).
Barbosa, M. J., Rocha, J. M. S., Tramper, J. & Wijffels, R. H. Acetate as a carbon source for hydrogen production by photosynthetic bacteria. J. Biotechnol. 85, 25–33 (2001).
Gosse, J. L. et al. Hydrogen production by photoreactive nanoporous latex coatings of nongrowing Rhodopseudomonas palustris CGA009. Biotechnol. Prog. 23, 124–130 (2007).
Rey, F. E., Heiniger, E. K. & Harwood, C. S. Redirection of metabolism for biological hydrogen production. Appl. Environ. Microbiol. 73, 1665–1671 (2007).
Golden, J. W. & Yoon, H. S. Heterocyst formation in Anabaena. Curr. Opin. Microbiol. 1, 623–629 (1998).
Cournac, L., Guedeney, G., Peltier, G. & Vignais, P. M. Sustained photoevolution of molecular hydrogen in a mutant of Synechocystis sp strain PCC 6803 deficient in the type I NADPH-dehydrogenase complex. J. Bacteriol. 186, 1737–1746 (2004).
Kosourov, S., Tsygankov, A., Seibert, M. & Ghirardi, M. L. Sustained hydrogen photoproduction by Chlamydomonas reinhardtii: effects of culture parameters. Biotechnol. Bioeng. 78, 731–740 (2002).
Kosourov, S., Patrusheva, E., Ghirardi, M. L., Seibert, M. & Tsygankov, A. A comparison of hydrogen photoproduction by sulfur-deprived Chlamydomonas reinhardtii under different growth conditions. J. Biotechnol. 128, 776–787 (2007).
Kruse, O., Rupprecht, J., Mussgnug, J. H., Dismukes, G. C. & Hankamer, B. Photosynthesis: a blueprint for solar energy capture and biohydrogen production technologies. Photochem. Photobiol. Sci. 4, 957–970 (2005).
Allahverdiyeva, Y. et al. Screening for biohydrogen production by cyanobacteria isolated from the Baltic Sea and Finnish lakes. Int. J. Hydrogen Energy 35, 1117–1127 (2010).
Zehr, J. P. et al. Unicellular cyanobacteria fix N2 in the subtropical North Pacific Ocean. Nature 412, 635–638 (2001).
Welsh, E. A. et al. The genome of Cyanothece 51142, a unicellular diazotrophic cyanobacterium important in the marine nitrogen cycle. Proc. Natl Acad. Sci. USA 105, 15094–15099 (2008).
Zehr, J. P., Methé, B. & Foster, R. in Biological Nitrogen Fixation, Sustainable Agriculture and the Environment Vol. 41 Current Plant Science and Biotechnology in Agriculture (eds Lin, M., Wang, Y.P., Tian, Z.X., Elmerich, C. & Newton, W. E.) 361–365 (Springer, 2005).
Schneegurt, M. A., Sherman, D. M. & Sherman, L. A. Growth, physiology and ultrastructure of the diazotrophic cyanobacterium, Cyanothece sp. strain ATCC 51142 in myxotrophic and chemotrophic cultures. J. Phycol. 33, 632–642 (1997).
Stockel, J. et al. Global transcriptomic analysis of Cyanothece 51142 reveals robust diurnal oscillation of central metabolic processes. Proc. Natl Acad. Sci. USA 105, 6156–6161 (2008).
Berman-Frank, I. et al. Segregation of nitrogen fixation and oxygenic photosynthesis in the marine cyanobacterium Trichodesmium. Science 294, 1534–1537 (2001).
Toepel, J., Welsh, E., Summerfield, T. C., Pakrasi, H. B. & Sherman, L. A. Differential transcriptional analysis of the cyanobacterium Cyanothece sp. strain ATCC 51142 during light-dark and continuous-light growth. J. Bacteriol. 190, 3904–3913 (2008).
Elvitigala, T., Stockel, J., Ghosh, B. K. & Pakrasi, H. B. Effect of continuous light on diurnal rhythms in Cyanothece sp. ATCC 51142. BMC Genomics 10, 226 (2009).
Dutta, D., De, D., Chaudhuri, S. & Bhattacharya, S. K. Hydrogen production by cyanobacteria. Microbial. Cell Factories 4, 36 (2005).
Feng, X. et al. Mixotrophic and photoheterotrophic metabolism in Cyanothece sp. ATCC 51142 under continuous light. Microbiology 156, 2566–2574 (2010).
Eisbrenner, G. & Evans, H. J. Aspects of hydrogen metabolism in nitrogen-fixing legumes and other plant-microbe associations. Annu. Rev. Plant Physiol. Plant Mol. Biol. 34, 105–136 (1983).
Liu, J. G., Bukatin, V. E. & Tsygankov, A. A. Light energy conversion into H2 by Anabaena variabilis mutant PK84 dense cultures exposed to nitrogen limitations. Int. J. Hydrogen Energy 31, 1591–1596 (2006).
Harwood, C. S. in Bioenergy (eds Harwood, C.S., Wall, J.D. & Demain, A.) 259–271 (ASM press, 2007).
Goldet, G. et al. Hydrogen production under aerobic conditions by membrane-bound hydrogenases from Ralstonia species. J. Am. Chem. Soc. 130, 11106–11113 (2008).
Montoya, J. P. et al. High rates of N2 fixation by unicellular diazotrophs in the oligotrophic Pacific Ocean. Nature 430, 1027–1032 (2004).
Mitsui, A. & Suda, S. Alternative and cyclic appearance of H2 and O2 photoproduction activities under nongrowing conditions in an aerobic nitrogen-fixing unicellular cyanobacterium Synechococcus sp. Curr. Microbiol. 30, 1–6 (1995).
Schutz, K. et al. Cyanobacterial H2 production—a comparative analysis. Planta 218, 350–359 (2004).
Happe, T., Schutz, K. & Bohme, H. Transcriptional and mutational analysis of the uptake hydrogenase of the filamentous cyanobacterium Anabaena variabilis ATCC 29413. J. Bacteriol. 182, 1624–1631 (2000).
Fay, P. Oxygen relations of nitrogen fixation in cyanobacteria. Microbiol. Rev. 56, 340–373 (1992).
Houchins, J. P. & Burris, R. H. Comparative characterization of two distinct hydrogenases from Anabaena sp. strain 7120. J. Bacteriol. 146, 215–221 (1981).
Tsygankov, A. A., Borodin, V. B., Rao, K. K. & Hall, D. O. H2 photoproduction by batch culture of Anabaena variabilis ATCC 29413 and its mutant PK84 in a photobioreactor. Biotechnol. Bioeng. 64, 709–715 (1999).
Tsygankov, A. A., Serebryakova, L. T., Rao, K. K. & Hall, D. O. Acetylene reduction and hydrogen photoproduction by wild-type and mutant strains of Anabaena at different CO2 and O2 concentrations. FEMS Microbiol. Lett. 167, 13–17 (1998).
Borodin, V. B., Tsygankov, A. A., Rao, K. K. & Hall, D. O. Hydrogen production by Anabaena variabilis PK84 under simulated outdoor conditions. Biotechnol. Bioeng. 69, 478–485 (2000).
Weyman, P. D., Pratte, B. & Thiel, T. Hydrogen production in nitrogenase mutants in Anabaena variabilis. FEMS Microbiol. Lett. 304, 55–61 (2010).
Asada, Y. & Kawamura, S. Aerobic hydrogen accumulation by a nitrogen-fixing cyanobacterium, Anabaena sp. Appl. Environ. Microbiol. 51, 1063–1066 (1986).
Xiankong, Z., Haskell, J. B., Tabita, F. R. & Baalen, C. V. Aerobic hydrogen production by the heterocystous cyanobacteria Anabaena spp. strains CA and 1F. J. Bacteriol. 156, 1118–1122 (1983).
Shestakov, S. V. & Mikheeva, L. E. Genetic control of hydrogen metabolism in cyanobacteria. Russian J. Genet 42, 1272–1284 (2006).
Kruse, O. et al. Improved photobiological H2 production in engineered green algal cells. J. Biol. Chem. 280, 34170–34177 (2005).
Min, H. T. & Sherman, L. A. Hydrogen production by the unicellular, diazotrophic cyanobacterium Cyanothece sp strain ATCC 51142 under conditions of continuous light. Appl. Environ. Microbiol. 76, 4293–4301 (2010).
Reddy, K. J., Haskell, J. B., Sherman, D. M. & Sherman, L. A. Unicellular, aerobic nitrogen-fixing cyanobacteria of the genus Cyanothece. J. Bacteriol. 175, 1284–1292 (1993).
Oda, Y. et al. Functional genomic analysis of three nitrogenase isozymes in the photosynthetic bacterium Rhodopseudomonas palustris. J. Bacteriol. 187, 7784–7794 (2005).
Acknowledgements
We thank Lawrence Page for the initial standardization of the hydrogen measurements assay and all other members of the Pakrasi and Sherman laboratories for collegial discussions. We also thank Dr. Teresa Thiel for providing us with the wild-type Anabaena variabilis strain. This work was supported by funding from DOE-BER (DE-FC02-07ER64694) and from the Consortium for Clean Coal Utilization at Washington University.
Author information
Authors and Affiliations
Contributions
A.B., J.S., L.A.S. and H.B.P. designed the experiments; A.B., J.S. and H.M. performed the experiments; A.B., J.S. and H.B.P. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Bandyopadhyay, A., Stöckel, J., Min, H. et al. High rates of photobiological H2 production by a cyanobacterium under aerobic conditions. Nat Commun 1, 139 (2010). https://doi.org/10.1038/ncomms1139
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms1139
This article is cited by
-
Quantitative insight into the metabolism of isoprene-producing Synechocystis sp. PCC 6803 using steady state 13C-MFA
Photosynthesis Research (2022)
-
Methanosarcina acetivorans contains a functional ISC system for iron-sulfur cluster biogenesis
BMC Microbiology (2020)
-
Aerobic nitrogen-fixing bacteria for hydrogen and ammonium production: current state and perspectives
Applied Microbiology and Biotechnology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.