Abstract
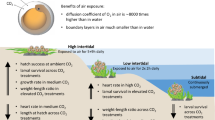
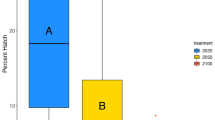
Ocean acidification negatively affects many marine species and is predicted to cause widespread changes to marine ecosystems. Similarly, freshwater ecosystems may potentially be affected by climate-change-related acidification; however, this has received far less attention. Freshwater fish represent 40% of all fishes, and salmon, which rear and spawn in freshwater, are of immense ecosystem, economical and cultural importance. In this study, we investigate the impacts of CO2-induced acidification during the development of pink salmon, in freshwater and following early seawater entry. At this critical and sensitive life stage, we show dose-dependent reductions in growth, yolk-to-tissue conversion and maximal O2 uptake capacity; as well as significant alterations in olfactory responses, anti-predator behaviour and anxiety under projected future increases in CO2 levels. These data indicate that future populations of pink salmon may be at risk without mitigation and highlight the need for further studies on the impact of CO2-induced acidification on freshwater systems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Dixson, D. L., Munday, P. L. & Jones, G. P. Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol. Lett. 13, 68–75 (2010).
Cripps, I. L., Munday, P. L. & McCormick, M. I. Ocean acidification affects prey detection by a predatory reef fish. PLoS ONE 6, e22736 (2011).
Munday, P. L. et al. Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc. Natl Acad. Sci. USA 106, 1848–1852 (2009).
Nilsson, G. E. et al. Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nature Clim. Change 2, 201–204 (2012).
Hamilton, T. J., Holcombe, A. & Tresguerres, M. CO2-induced ocean acidification increases anxiety in rockfish via alteration of GABAA receptor functioning. Proc. R. Soc. B 281, 20132509 (2014).
Jutfelt, F., de Souza, K. B., Vuylsteke, A. & Sturve, J. Behavioural disturbances in a temperate fish exposed to sustained high-CO2 levels. PLoS ONE 8, e65825 (2013).
Van de Waal, D. B., Verschoor, A. M., Verspagen, J. M., van Donk, E. & Huisman, J. Climate-driven changes in the ecological stoichiometry of aquatic ecosystems. Front. Ecol. Environ. 8, 145–152 (2009).
Dudgeon, D. et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 81, 163–182 (2006).
Willson, M. F. & Halupka, K. C. Anadromous fish as keystone species in vertebrate communities. Conserv. Biol. 9, 489–497 (1995).
Quinn, T. P. The Behavior and Ecology of Pacific Salmon and Trout (Univ. Washington Press, 2005).
Stefansson, S. O., Björnsson, B. T., Ebbesson, L. O. E. & McCormick, S. D. in Fish Larval Physiology (eds Finn, R. N. & Kapoor, B. G.) 639–681 (Science, 2008).
Neave, F., Ishida, T. & Murai, S. Salmon of the North Pacific Ocean. Part VI. Pink salmon in offshore waters. Int. North Pac. Fish. Comm. Bull. 22, 1–33 (1967).
Grant, A. et al. Growth and ionoregulatory ontogeny of wild and hatchery-raised juvenile pink salmon (Oncorhynchus gorbuscha). Can. J. Zool. 87, 221–228 (2009).
Heard, W. R. in Pacific Salmon Life Histories (eds Groot, C. & Margolis, L.) 319–377 (UBC Press, 1991).
Parker, R. R. Estimations of ocean mortality rates for Pacific salmon (Oncorhynchus). J. Fish. Res. Board Can. 19, 561–589 (1962).
Durkin, J. T. in Estuarine Comparisons (ed. Kennedy, V. S.) 365–376 (Academic Press, 1982).
Healey, M. C. Timing and relative intensity of size-selective mortality of juvenile chum salmon (Oncorhynchus keta) during early sea life. Can. J. Fish. Aquat. Sci. 39, 952–957 (1982).
Cole, J. J., Caraco, N. F., Kling, G. W. & Kratz, T. K. Carbon dioxide supersaturation in the surface waters of lakes. Science 265, 1568–1570 (1994).
Raymond, P. A., Caraco, N. F. & Cole, J. J. Carbon dioxide concentration and atmospheric flux in the Hudson River. Estuaries 20, 381–390 (1997).
Reum, J. C. et al. Seasonal carbonate chemistry covariation with temperature, oxygen, and salinity in a fjord estuary: Implications for the design of ocean acidification experiments. PLoS ONE 9, e89619 (2014).
Prut, L. & Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 463, 3–33 (2003).
Tseng, Y. et al. CO2-driven seawater acidification differentially affects development and molecular plasticity along life history of fish (Oryzias latipes). Comp. Biochem. Physiol. A 165, 119–130 (2013).
Baumann, H., Talmage, S. C. & Gobler, C. J. Reduced early life growth and survival in a fish in direct response to increased carbon dioxide. Nature Clim. Change 2, 38–41 (2012).
Munday, P. L., Donelson, J. M., Dixson, D. L. & Endo, G. G. K. Effects of ocean acidification on the early life history of a tropical marine fish. Proc. R. Soc. B 276, 3275–3283 (2009).
Pepin, P. Effect of temperature and size on development, mortality and survival rates of the pelagic early life history stages of marine fish. Can. J. Fish. Aquat. Sci. 58, 503–518 (1991).
Hendry, A. P., Day, T. & Cooper, A. B. Optimal size and number of propagules: Allowance for discrete stages and effects of maternal size on reproductive output and offspring fitness. Am. Nature 157, 387–407 (2001).
Munday, P. L. et al. Replenishment of fish populations is threatened by ocean acidification. Proc. Natl Acad. Sci. USA 107, 12930–12934 (2010).
Ferrari, M. C. O. et al. Putting prey and predator into the CO2 equation: Qualitative and quantitative effects of ocean acidification on predator–prey interactions. Ecol. Lett. 14, 1143–1148 (2011).
Leduc, A. O., Kelly, J. M. & Brown, G. E. Detection of conspecific alarm cues by juvenile salmonids under neutral and weakly acidic conditions: Laboratory and field tests. Oecologia 139, 318–324 (2004).
Brown, G. E., Adrian, J. C. Jr, Lewis, M. G. & Tower, J. M. The effects of reduced pH on chemical alarm signalling in ostariophysan fishes. Can. J. Fish. Aquat. Sci. 59, 1331–1338 (2002).
Leduc, A. O., Roh, E. & Brown, G. E. Effects of acid rainfall on juvenile Atlantic salmon (Salmo salar) antipredator behaviour: Loss of chemical alarm function and potential survival consequences during predation. Mar. Freshwat. Res. 60, 1223–1230 (2009).
Lemly, D. A. & Smith, R. J. F. Effects of acute exposure to acidified water on the behavioral response of fathead minnows, Pimephales promelas, to chemical feeding stimuli. Aquat. Toxicol. 6, 25–36 (1985).
Miller, G. M., Watson, S. A., Donelson, J. M., McCormick, M. I. & Munday, P. L. Parental environment mediates impacts of increased carbon dioxide on a coral reef fish. Nature Clim. Change 2, 858–861 (2012).
Welch, M. J., Watson, S. A., Welsh, J. Q., McCormick, M. I. & Munday, P. L. Effects of elevated CO2 on fish behaviour undiminished by transgenerational acclimation. Nature Clim. Change 4, 1086–1089 (2014).
Moore, A. An electrophysiological study on the effects of pH on olfaction in mature male Atlantic salmon (Salmo salar) parr. J. Fish Biol. 45, 493–502 (1994).
Shoji, T. et al. Amino acids dissolved in stream water as possible home stream odorants for masu salmon. Chem. Senses 25, 533–540 (2000).
Shoji, T., Yamamoto, Y., Nishikawa, D., Kurihara, K. & Ueda, H. Amino acids in stream water are essential for salmon homing migration. Fish Physiol. Biochem. 28, 249–251 (2003).
Kitamura, S. & Ikuta, K. Acidification severely suppresses spawning of hime salmon (land-locked sockeye salmon, Oncorhynchus nerka). Aquat. Toxicol. 51, 107–113 (2000).
Kitamura, S. & Ikuta, K. Effects of acidification on salmonid spawning behavior. Wat. Air Soil Pollut. 130, 875–880 (2001).
Ikuta, K., Munakata, A., Aida, K., Amano, M. & Kitamura, S. Effects of low pH on upstream migratory behavior in land-locked sockeye salmon Oncorhynchus nerka. Wat. Air Soil Pollut. 130, 99–106 (2001).
Leduc, A. O., Munday, P. L., Brown, G. E. & Ferrari, M. C. Effects of acidification on olfactory-mediated behaviour in freshwater and marine ecosystems: A synthesis. Phil. Trans. R. Soc. B 368, 20120447 (2013).
López-Patiño, M. A., Yu, L., Cabral, H. & Zhdanova, I. V. Anxiogenic effects of cocaine withdrawal in zebrafish. Physiol. Behav. 93, 160–171 (2008).
Wong, K. et al. Analyzing habituation responses to novelty in zebrafish (Danio rerio). Behav. Brain Res. 208, 450–457 (2010).
López Patiño, M. A., Yu, L., Yamamoto, B. K. & Zhdanova, I. V. Gender differences in zebrafish responses to cocaine withdrawal. Physiol. Behav. 95, 36–47 (2008).
Raymond, P. A. et al. Global carbon dioxide emissions from inland waters. Nature 503, 355–359 (2013).
Park, P. K., Gordon, L. I., Hager, S. W. & Cissell, M. C. Carbon dioxide partial pressure in the Columbia River. Science 166, 867–868 (1969).
Evans, W., Hales, B. & Strutton, P. G. Seasonal cycle of surface ocean pCO2 on the Oregon shelf. J. Geophys. Res. 116, C05012 (2011).
Hales, B., Takahashi, T. & Bandstra, L. Atmospheric CO2 uptake by a coastal upwelling system. Glob. Biogeochem. Cycles 19, GB1009 (2005).
Frieder, C. A., Nam, S. H., Martz, T. R. & Levin, L. A. High temporal and spatial variability of dissolved oxygen and pH in a nearshore California kelp forest. Biogeosciences 9, 3917–3930 (2012).
Marliave, J. B., Gibbs, C. J., Gibbs, D. M., Lamb, A. O. & Young, S. J. in Biodiversity Loss in a Changing Planet (eds Grillo, O. & Venora, G.) 49–74 (In Tech, 2011).
Healy, T. M. & Schulte, P. M. Thermal acclimation is not necessary to maintain a wide thermal breadth of aerobic scope in the common killifish (Fundulus heteroclitus). Physiol. Biochem. Zool. 85, 107–119 (2012).
Reidy, S. P., Nelson, J. A., Tang, Y. & Kerr, S. R. Post-exercise metabolic rate in Atlantic cod and its dependence upon the method of exhaustion. J. Fish Biol. 47, 377–386 (1995).
Sylvestre, E. L., Lapointe, D., Dutil, J. D. & Guderley, H. Thermal sensitivity of metabolic rates and swimming performance in two latitudinally separated populations of cod, Gadus morhuaa L. J. Comp. Physiol. B 177, 447–460 (2007).
Killen, S. S., Costa, I., Brown, J. A. & Gamperl, A. K. Little left in the tank: Metabolic scaling in marine teleosts and its implications for aerobic scope. Proc. R. Soc. B 274, 431–438 (2007).
Wieser, W. Developmental and metabolic constraints of the scope for activity in young rainbow trout (Salmo gairdneri). J. Exp. Biol. 118, 133–142 (1985).
Peake, S. J. & Farrell, A. P. Locomotory behaviour and post-exercise physiology in relation to swimming speed, gait transition and metabolism in free-swimming smallmouth bass (Micropterus dolomieu). J. Exp. Biol. 207, 1563–1575 (2004).
Clark, T. D., Sandblom, E. & Jutfelt, F. Aerobic scope measurements of fishes in an era of climate change: Respirometry, relevance and recommendations. J. Exp. Biol. 216, 2771–2782 (2013).
Nendick, L. et al. Swimming performance and associated ionic disturbance of juvenile pink salmon Oncorhynchus gorbuscha determined using different acceleration profiles. J. Fish Biol. 75, 1626–1638 (2009).
McDonald, D. G., McFarlane, W. J. & Milligan, C. L. Anaerobic capacity and swim performance of juvenile salmonids. Can. J. Fish. Aquat. Sci. 55, 1198–1207 (1998).
McFarlane, W. J. & McDonald, D. G. Relating intramuscular fuel use to endurance in juvenile rainbow trout. Physiol. Biochem. Zool. 75, 250–259 (2002).
Gerlach, G., Atema, J., Kingsford, M. J., Black, K. P. & Miller-Sims, V. Smelling home can prevent dispersal of reef fish larvae. Proc. Natl Acad. Sci. USA 104, 858–863 (2007).
Acknowledgements
We thank D. Ewart and the staff at Quinsam River Hatchery for providing us with pink salmon embryos and S. Balshine for her comments on the manuscript. Special thanks to G. Fullerton, P. Tamkee, B. Gillespie and the UBC Comphy group for their help and support throughout this project. The project was financially supported by Natural Sciences and Engineering Research Council (NSERC) Discovery grants to C.J.B. and T.J.H. and a NSERC Accelerator Supplement to C.J.B.
Author information
Authors and Affiliations
Contributions
M.O. and C.J.B. devised the study. M.O., C.J.B., J.E., T.J.H. and S.-S.Y. designed the experiments. M.O., T.J.H., J.E., E.M.L., J.G., A.J. and J.L. conducted the experiments. M.O., E.M.L., J.E. and T.J.H. developed equipment. M.O. and E.M.L. collected water samples and conducted water analyses. M.O. and C.J.B. wrote the manuscript. M.O., C.J.B., T.J.H., J.E., S.-S.Y. and D.A.C. contributed to intellectual input and edited this manuscript. All authors approved this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Ou, M., Hamilton, T., Eom, J. et al. Responses of pink salmon to CO2-induced aquatic acidification. Nature Clim Change 5, 950–955 (2015). https://doi.org/10.1038/nclimate2694
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nclimate2694
This article is cited by
-
Repeated microdoses of LSD do not alter anxiety or boldness in zebrafish
Scientific Reports (2024)
-
Examining behavioural test sensitivity and locomotor proxies of anxiety-like behaviour in zebrafish
Scientific Reports (2023)
-
A review of marine stressors impacting Atlantic salmon Salmo salar, with an assessment of the major threats to English stocks
Reviews in Fish Biology and Fisheries (2022)
-
Climate change threatens Chinook salmon throughout their life cycle
Communications Biology (2021)
-
The ontogeny of Na+ balance during rapid smoltification in pink salmon (Oncorhynchus gorbuscha)
Journal of Comparative Physiology B (2021)