Abstract
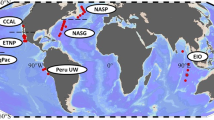
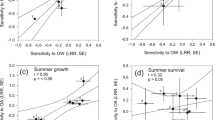
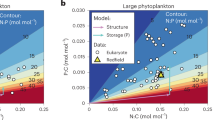
Phytoplankton are the basis of marine food webs, and affect biogeochemical cycles. As CO2 levels increase, shifts in the frequencies and physiology of ecotypes within phytoplankton groups will affect their nutritional value and biogeochemical function. However, studies so far are based on a few representative genotypes from key species. Here, we measure changes in cellular function and growth rate at atmospheric CO2 concentrations predicted for the year 2100 in 16 ecotypes of the marine picoplankton Ostreococcus. We find that variation in plastic responses among ecotypes is on par with published between-genera variation, so the responses of one or a few ecotypes cannot estimate changes to the physiology or composition of a species under CO2 enrichment. We show that ecotypes best at taking advantage of CO2 enrichment by changing their photosynthesis rates most should increase in relative fitness, and so in frequency in a high-CO2 environment. Finally, information on sampling location, and not phylogenetic relatedness, is a good predictor of ecotypes likely to increase in frequency in this system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Vaulot, D., Eikrem, W., Viprey, M. & Moreau, H. The diversity of small eukaryotic phytoplankton <3 μm in marine ecosystems. FEMS Microbiol. Rev. 32, 795–820 (2008).
Doney, S. C., Fabry, V. J., Feely, R. A. & Kleypas, J. A. Ocean acidification: The other CO2 problem. Annu. Rev. Marine. Sci. 1, 169–192 (2009).
Riebesell, U. et al. Enhanced biological carbon consumption in a high CO2 ocean. Nature 450, 545–548 (2007).
Sterner, R., Hagemeier, D., Smith, W. & Smith, R. Phytoplankton nutrient limitation and food quality for Daphnia. Limnol. Oceanogr. 38, 857–871 (1993).
Hoppe, C. J. M., Langer, G. & Rost, B. Emiliania huxleyi shows identical responses to elevated pCO2 in TA and DIC manipulations. J. Exp. Mar. Biol. Ecol. 406, 54–62 (2010).
Kranz, S. A., Eichner, M. & Rost, B. Interactions between CCM and N2 fixation in Trichodesmium. Photosynth. Res. 109, 73–84 (2010).
Rossoll, D. et al. Ocean acidification-induced food quality deterioration constrains trophic transfer. PLoS ONE 7, e34737 (2012).
Langer, G., Nehrke, G., Probert, I., Ly, J. & Ziveri, P. Strain-specific responses of Emiliania huxleyi to changing seawater carbonate chemistry. Biogeosciences 6, 2637–2646 (2009).
Fabry, V. J., Seibel, B. A., Feely, R. A. & Orr, J. C. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES. J. Mar. Sci. 65, 414–432 (2008).
Le Quere, C. et al. Ecosystem dynamics based on plankton functional types for global ocean biogeochemistry models. Glob. Change Biol. 11, 2016–2040 (2005).
Courties, C. et al. Smallest eukaryotic organism. Nature 370, 255–255 (1994).
Rodrı´guez, F. et al. Ecotype diversity in the marine picoeukaryoteOstreococcus (Chlorophyta, Prasinophyceae). Environ. Microbiol. 7, 853–859 (2005).
Demir-Hilton, E. et al. Global distribution patterns of distinct clades of the photosynthetic picoeukaryote Ostreococcus. ISME J. 5, 1095–1107 (2011).
Worden, A., Nolan, J. & Palenik, B. Assessing the dynamics and ecology of marine picophytoplankton: The importance of the eukaryotic component. Limnol. Oceanogr. 49, 168–179 (2004).
Elser, J., Hayakawa, K. & Urabe, J. Nutrient limitation reduces food quality for zooplankton: Daphnia response to seston phosphorus enrichment. Ecology 82, 898–903 (2001).
Egleston, E. S., Sabine, C. L. & Morel, F. M. M. Revelle revisited: Buffer factors that quantify the response of ocean chemistry to changes in DIC and alkalinity. Glob. Biogeochem. Cycles 24, GB1002 (2010).
Collins, S. & Bell, G. Phenotypic consequences of 1,000 generations of selection at elevated CO2 in a green alga. Nature 431, 566–569 (2004).
Fu, F.-X., Warner, M. E., Zhang, Y., Feng, Y. & Hutchins, D. A. Effects of increased temperature and CO2 on photosynthesis, growth, and elemental ratios in marine Synechococcus and Prochlorococcus (Cyanobacteria). J. Phycol. 43, 485–496 (2007).
Fu, F. X. et al. Interactions between changing pCO2, N2 fixation, and Fe limitation in the marine unicellular cyanobacterium Crocosphaera. Limnol. Oceanogr. 6, 2472–2484 (2008).
Sterner, R. & Elser, J. Ecological Stoichiometry: The Biology of Elements from co2 Molecules to the Biosphere (Princeton Univ. Press, 2002).
Lenski, R. E., Rose, M. R., Simpson, S. C. & Tadler, S. C. Long-term experimental evolution in Escherichia coli. I. Adaptation and divergence during 2,000 generations. Am. Natural. 138, 1315–1341 (1991).
Collins, S. Many possible worlds: Expanding the ecological scenarios in experimental evolution. Evol. Biol. 38, 3–14 (2011).
Tortell, P. D. et al. CO2 sensitivity of Southern Ocean phytoplankton. Geophys. Res. Lett. 35, L04605 (2008).
Copin-Montégut, C., Bégovic, M. & Merlivat, L. Variability of the partial pressure of CO2 on diel to annual time scales in the Northwestern Mediterranean Sea. Mar. Chem. 85, 169–189 (2004).
Takahashi, T. et al. Corrigendum to ‘Climatological mean and decadal change in surface ocean pCO2, and net sea–air CO2 flux over the global oceans’ (Deep Sea Res. II 56, 554–577 (2009)). Deep-Sea Res. I 56, 2075–2076 (2009).
Mueller, M. N., Schulz, K. G. & Riebesell, U. Effects of long-term high CO2 exposure on two species of coccolithophores. Biogeosciences 7, 1109–1116 (2010).
Van de Waal, D. B. et al. Reversal in competitive dominance of a toxic versus non-toxic cyanobacterium in response to rising CO2 . ISME J. 5, 1438–1450 (2011).
Keller, M. D., Selvin, R. C., Claus, W. & Guillard, R. Media for the culture of oceanic ultra phytoplankton. J. Phycol. 23, 633–638 (1987).
Lewis, E. & Wallace, D. Program Developed for the CO 2 System Calculations (Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy, 1998).
Rokitta, S. & Rost, B. Effects of CO2 and their modulation by light in the life-cycle stages of the coccolithophore Emiliania huxleyi. Limnol. Oceanogr. 57, 607–618 (2012).
Levitan, O. et al. Elevated CO2 enhances nitrogen fixation and growth in the marine cyanobacterium Trichodesmium. Glob. Change Biol. 13, 531–538 (2007).
Barcelos e Ramos, J., Biswas, H., Schulz, K. G., LaRoche, J. & Riebesell, U. Effect of rising atmospheric carbon dioxide on the marine nitrogen fixer Trichodesmium. Glob. Biogeochem. Cycles 21, GB2028 (2007).
Hutchins, D., Fu, F., Zhang, Y. & Warner, M. CO2 control of Trichodesmium N2 fixation, photosynthesis, growth rates, and elemental ratios: Implications for past, present, and future ocean biogeochemistry. Limnol. Oceanogr. 52, 1293–1304 (2007).
Kranz, S., Sultemeyer, D., Richter, K. U. & Rost, B. Carbon acquisition in Trichodesmium: The effect of pCO2 and diurnal changes. Limnol. Oceanogr. 54, 548–559 (2009).
Trimborn, S. et al. Inorganic carbon acquisition in potentially toxic and non-toxic diatoms: The effect of pH-induced changes in seawater carbonate chemistry. Phys. Plant 133, 92–105 (2008).
Wu, Y., Gao, K. & Riebesell, U. CO2-induced seawater acidification affects physiological performance of the marine diatom Phaeodactylum tricornutum. Biogeosciences 7, 2915–2923 (2010).
Hu, H. & Gao, K. Impacts of CO2 enrichment on growth and photosynthesis in freshwater and marine diatoms. Chin. J. Oceanol. Limnol. 26, 407–414 (2009).
Langer, G. et al. Species-specific responses of calcifying algae to changing seawater carbonate chemistry. Geochem. Geophys. Geosyst. 7, Q09006 (2006).
Lohbeck, K. T., Riebesell, U. & Reusch, T. B. H. Adaptive evolution of a key phytoplankton species to ocean acidification. Nature Geosci. 5, 346–351 (2012).
Feng, Y. et al. Interactive effects of increased pCO2, temperature and irradiance on the marine coccolithophore Emiliania huxleyi (Prymnesiophyceae). Eur. J. Phycol. 43, 87–98 (2008).
Acknowledgements
This study was conducted at the University of Edinburgh (UK) and the Alfred Wegener Institute for Polar and Marine Research (Germany). The research was supported by a Royal Society (UK) University Research Fellowship (S.C.), the European Research Council (ERC) under the European Community’s Seventh Framework Programme (FP7/2007-2013), ERC grant agreement 205150 (B.R.) and a Scottish Universities Life Science Alliance scholarship (E.S.). We thank M. Allen and ASSEMBLE (Association of European Marine Biology Laboratories) Roscoff for providing the Ostreococcus ecotypes; H. Kuehne, S. Reece and T. Reusch for advice on the manuscript; J. Raven for advice concerning the experiments; and S. Rokitta, K-U. and U. Richter for assistance in the laboratory at the AWI.
Author information
Authors and Affiliations
Contributions
E.S. designed and performed the experiments, analysed data and wrote the manuscript. S.C. designed the experiments, analysed data, wrote the manuscript and supervised laboratory work. B.R. supervised the laboratory work at the Alfred-Wegener-Institute and contributed to the manuscript. A.J.M. contributed to the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1242 kb)
Rights and permissions
About this article
Cite this article
Schaum, E., Rost, B., Millar, A. et al. Variation in plastic responses of a globally distributed picoplankton species to ocean acidification. Nature Clim Change 3, 298–302 (2013). https://doi.org/10.1038/nclimate1774
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nclimate1774
This article is cited by
-
Genetic assimilation of ancestral plasticity during parallel adaptation to zinc contamination in Silene uniflora
Nature Ecology & Evolution (2023)
-
Multivariate trait analysis reveals diatom plasticity constrained to a reduced set of biological axes
ISME Communications (2021)
-
Evapotranspiration depletes groundwater under warming over the contiguous United States
Nature Communications (2020)
-
Acidification diminishes diatom silica production in the Southern Ocean
Nature Climate Change (2019)
-
Utilization of waste plastic oil in diesel engines: a review
Reviews in Environmental Science and Bio/Technology (2019)