Abstract
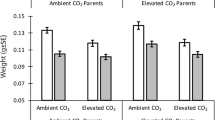
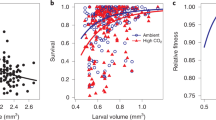
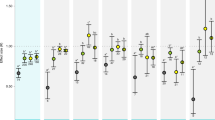
Carbon dioxide concentrations in the surface ocean are increasing owing to rising CO2 concentrations in the atmosphere1. Higher CO2 levels are predicted to affect essential physiological processes of many aquatic organisms2,3, leading to widespread impacts on marine diversity and ecosystem function, especially when combined with the effects of global warming4,5,6. Yet the ability for marine species to adjust to increasing CO2 levels over many generations is an unresolved issue. Here we show that ocean conditions projected for the end of the century (approximately 1,000 μatm CO2 and a temperature rise of 1.5–3.0 °C) cause an increase in metabolic rate and decreases in length, weight, condition and survival of juvenile fish. However, these effects are absent or reversed when parents also experience high CO2 concentrations. Our results show that non-genetic parental effects can dramatically alter the response of marine organisms to increasing CO2 and demonstrate that some species have more capacity to acclimate to ocean acidification than previously thought.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Doney, S. C. The growing human footprint on coastal and open-ocean biogeochemistry. Science 328, 1512–1516 (2010).
Pörtner, H. O., Langenbuch, M. & Reipschlager, A. Biological impact of elevated ocean CO2 concentrations: Lessons from animal physiology and earth history. J. Oceanogr. 60, 705–718 (2004).
Melzner, F. et al. Physiological basis for high CO2 tolerance in marine ectothermic animals: Pre-adaptation through lifestyle and ontogeny? Biogeoscience 6, 2313–2331 (2009).
Hoegh-Guldberg, O. et al. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742 (2007).
Pörtner, H. O. & Farrell, A. P. Ecology: Physiology and climate change. Science 322, 690–692 (2008).
Fabry, V. J., Seibel, B. A., Feely, R. A. & Orr, J. C. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci. 65, 414–432 (2008).
Rosa, R. & Seibel, B. A. Synergistic effects of climate-related variables suggest future physiological impairment in a top oceanic predator. Proc. Natl Acad. Sci. USA 105, 20776–20780 (2008).
Pörtner, H. O. & Knust, R. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315, 95–97 (2007).
Eliason, E. J. et al. Differences in thermal tolerance among sockeye salmon populations. Science 332, 109–112 (2011).
Nilsson, G. E., Crawley, N., Lunde, I. G. & Munday, P. L. Elevated temperature reduces the respiratory scope of coral reef fishes. Glob. Change Biol. 15, 1405–1412 (2009).
Pandolfi, J. M., Connolly, S. R., Marshall, D. J. & Cohen, A. L. Projecting coral reef futures under global warming and ocean acidification. Science 333, 418–422 (2011).
Pörtner, H. O. et al. in Ocean Acidification (eds Gattuso, J-P. & Hansson, L.) (Oxford Univ. Press, 2011).
Marshall, D. J. & Morgan, S. G. Ecological and evolutionary consequences of linked life-history stages in the sea. Curr. Biol. 21, R718-R725 (2011).
Parker, L. M. et al. Adult exposure influences offspring response to ocean acidification in oysters. Glob. Change Biol. 18, 82–92 (2012).
Donelson, J. M., Munday, P. L. & McCormick, M.I. Rapid transgenerational acclimation of a tropical reef fish to climate change. Nature Clim. Change 2, 30–32 (2012).
Salinas, S. & Munch, S.B. Thermal legacies: Transgenerational effects of temperature on growth in a vertebrate. Ecol. Lett. 15, 159–163 (2012).
Meehl, G. A. et al. in IPCC Climate Change 2007: The Physical Science Basis (eds Solomon, S. et al.) (Cambridge Univ. Press, 2007).
Poloczanska, E. S. et al. Climate change and Australian marine life. Oceanogr. Mar. Biol. Annu. Rev. 45, 407–478 (2007).
Nowicki, J. P., Miller, G. M. & Munday, P. L. Interactive effects of elevated temperature and CO2 on foraging behavior of juvenile coral reef fish. J. Exp. Mar. Biol. Ecol. 412, 46–51 (2012).
Bonduriansky, R. & Day, T. Nongenetic inheritance and its evolutionary implications. Annu. Rev. Ecol. Evol. Syst. 40, 103–125 (2009).
Donelson, J. M., Munday, P. L. & McCormick, M. I. Parental effects on offspring life histories: when are they important? Biol. Lett. 5, 262–265 (2009).
Bernardo, J. Maternal effects in animal ecology. Am. Zool. 36, 83–105 (1996).
Kurihara, H. Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Mar. Ecol. Prog. Ser. 373, 275–284 (2008).
Hendriks, I. E., Duarte, C. M. & Alvarez, M. Vulnerability of marine biodiversity to ocean acidification: A meta-analysis. Estuar. Coast. Shelf Sci. 86, 157–164 (2010).
Kroeker, K. J., Kordas, R. L., Crim, R. N. & Singh, G. G. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol. Lett. 13, 1419–1434 (2010).
Baumann, H., Talmage, S. C. & Gobler, C. J. Reduced early life growth and survival in a fish in direct response to increased carbon dioxide. Nature Clim. Change 2, 38–41 (2012).
Frommel, A. Y. et al. Severe tissue damage in Atlantic cod larvae under increasing ocean acidification. Nature Clim. Change 2, 42–46 (2012).
Jablonka, E. & Raz, G. Transgenerational epigenetic inheritance: Prevalence, mechanisms, and implications for the study of heredity and evolution. Q. Rev. Biol. 84, 131–176 (2009).
Deigweiher, K., Koschnick, N., Pörtner, H. O. & Lucassen, M. Acclimation of ion regulatory capacities in gills of marine fish under environmental hypercapnia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R1660-R1670 (2008).
Green, B. S. Maternal effects in fish populations. Adv. Mar. Biol. 54, 1–105 (2008).
Ishimatsu, A., Hayashi, M. & Kikkawa, T. Fishes in high-CO2, acidified oceans. Mar. Ecol. Prog. Ser. 373, 295–302 (2008).
Munday, P. L., Donelson, J. M., Dixson, D. L. & Endo, G. G. K. Effects of ocean acidification on the early life history of a tropical marine fish. Proc. R. Soc. B 276, 3275–3283 (2009).
Munday, P.L., Hernaman, V., Dixson, D. L. & Thorrold, S.R. Effect of ocean acidification on otolith development in larvae of a tropical marine fish. Biogeosciences 8, 1631–1641 (2011).
Acknowledgements
We thank staff of James Cook University’s Marine Aquarium Facility, R. De Nys for logistical support and J. L. Rummer for comments on the manuscript. The project was financially supported by the Australian Research Council (P.L.M), ARC Centre of Excellence for Coral Reef Studies (P.L.M. and M.I.M.) and Sea World Research and Rescue Foundation (G.M.M.). This project was completed under JCU Ethics A1427.
Author information
Authors and Affiliations
Contributions
G.M.M. and P.L.M. designed the experiments. G.M.M. carried out all experimentation and analysed raw data. S-A.W. and G.M.M. collected and analysed seawater chemistry parameters. P.L.M., G.M.M., J.M.D., S-A.W. and M.I.M. wrote the article. All authors contributed intellectual input, read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 0 kb)
Rights and permissions
About this article
Cite this article
Miller, G., Watson, SA., Donelson, J. et al. Parental environment mediates impacts of increased carbon dioxide on a coral reef fish. Nature Clim Change 2, 858–861 (2012). https://doi.org/10.1038/nclimate1599
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nclimate1599
This article is cited by
-
Transgenerational exposure to ocean acidification impacts the hepatic transcriptome of European sea bass (Dicentrarchus labrax)
BMC Genomics (2023)
-
The extensive transgenerational transcriptomic effects of ocean acidification on the olfactory epithelium of a marine fish are associated with a better viral resistance
BMC Genomics (2022)
-
Microbiota mediated plasticity promotes thermal adaptation in the sea anemone Nematostella vectensis
Nature Communications (2022)
-
Nitrate enrichment has lineage specific effects on Pocillopora acuta adults, but no transgenerational effects in planulae
Coral Reefs (2022)
-
Impacts of hypoxic events surpass those of future ocean warming and acidification
Nature Ecology & Evolution (2021)