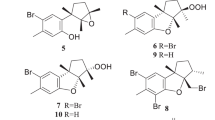
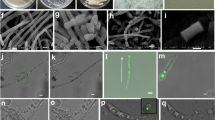
Abstract
Natural products are both a fundamental source of new chemical diversity and an integral component of today's pharmaceutical compendium. Yet interest in natural-product drug discovery has waned, in part owing to diminishing returns from traditional resources such as soil bacteria. The oceans cover 70% of the Earth's surface and harbor most of the planet's biodiversity. Although marine plants and invertebrates have received considerable attention as a resource for natural-product discovery, the microbiological component of this diversity remains relatively unexplored. Recent studies have revealed that select groups of marine actinomycetes are a robust source of new natural products. Members of the genus Salinispora have proven to be a particularly rich source of new chemical structures, including the potent proteasome inhibitor salinosporamide A, and other distinct groups are yielding new classes of terpenoids, amino acid–derived metabolites and polyene macrolides. The continued development of improved cultivation methods and technologies for accessing deep-sea environments promises to provide access to this significant new source of chemical diversity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Newman, D.J., Cragg, G.M. & Snader, K.M. Natural products as sources of new drugs over the period 1981–2002. J. Nat. Prod. 66, 1022–1037 (2003).
Koehn, F.E. & Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 4, 206–220 (2005).
Clardy, J. & Walsh, C. Lessons from natural molecules. Nature 432, 829–837 (2004).
Walsh, C. Where will new antibiotics come from? Nat. Rev. Microbiol. 1, 65–70 (2003).
Goodfellow, M. & Haynes, J.A. in Biological, Biochemical, and Biomedical Aspects of Actinomycetes (eds. Ortiz-Ortiz, L., Bojalil, L.F. & Yakoleff, V.) 453–472 (Academic, Orlando, Florida, 1984).
ZoBell, C.E. Marine Microbiology (Chronica Botanica Co., Waltham, Massachusetts, 1946).
Amann, R.I., Ludwig, W. & Schleifer, K.-H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59, 143–169 (1995).
Venter, J.C. et al. Environmental genome shotgun sequencing of the Sargasso Sea. Science 304, 66–74 (2004).
Lam, K.S. Discovery of novel metabolites from marine actinomycetes. Curr. Opin. Microbiol. 9, 245–251 (2006).
Bérdy, J. Bioactive microbial metabolites. J. Antibiot. (Tokyo) 58, 1–26 (2005).
Weyland, H. Actinomycetes in North Sea and Atlantic Ocean sediments. Nature 223, 858 (1969).
Pathom-aree, W. et al. Diversity of actinomycetes isolated from the Challenger Deep sediment (10,898 m) from the Mariana Trench. Extremophiles 10, 181–189 (2006).
Helmke, E. & Weyland, H. Rhodococcus marinonascens sp. nov., an actinomycete from the sea. Int. J. Syst. Bacteriol. 34, 127–138 (1984).
Han, S.K., Nedashkovzkaya, O.I., Mikhailov, V.V., Kim, S.B. & Bae, K.S. Salinibacterium amurkyense gen. nov., sp. nov., a novel genus of the family Microbacteriaceae from the marine environment. Int. J. Syst. Evol. Microbiol. 53, 2061–2066 (2003).
Yi, H., Schumann, P., Sohn, K. & Chun, J. Serinicoccus marinus gen. nov., sp. nov., a novel actinomycete with L-ornithine and L-serine in the pedtidoglycan. Int. J. Syst. Evol. Microbiol. 54, 1585–1589 (2004).
Maldonado, L. et al. Salinispora gen nov., a home for obligate marine actinomycetes belonging to the family Micromonosporaceae. Int. J. Sys. Evol. Microbiol. 55, 1759–1766 (2005).
Jensen, P.R., Dwight, R. & Fenical, W. Distribution of actinomycetes in near-shore tropical marine sediments. Appl. Environ. Microbiol. 57, 1102–1108 (1991).
Mincer, T.J., Jensen, P.R., Kauffman, C.A. & Fenical, W. Widespread and persistent populations of a major new marine actinomycete taxon in ocean sediments. Appl. Environ. Microbiol. 68, 5005–5011 (2002).
Feling, R.H. et al. Salinosporamide A: a highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus Salinispora. Angew. Chem. Int. Edn. Engl. 42, 355–357 (2003).
Omura, S. et al. Lactacystin, a novel microbial metabolite, induces neuritogenesis of neuroblastoma cells. J. Antibiot. (Tokyo) 44, 113–116 (1991).
Chauhan, D. et al. A novel orally active proteasome inhibitor induces apoptosis in multiple myelome cells with mechanisms distinct from bortezomib. Cancer Cell 8, 407–419 (2005).
Groll, M., Huber, R. & Potts, B.C.M. Crystal structure of salinosporamide A (NPI-0052) and B (NPI-0047) in complex with the 20S proteasome reveal important consequences of beta-lactone ring opening and a mechanism for irreversible binding. J. Am. Chem. Soc. 128, 5136–5141 (2006).
Macherla, V.R. et al. Structure-activity relationship studies of Salinosporamide A (NPI-0052), a novel marine derived proteasome inhibitor. J. Med. Chem. 48, 3684–3687 (2005).
Williams, P.G. et al. New cytotoxic salinosporamides from the marine actinomycete Salinispora tropica. J. Org. Chem. 70, 6196–6203 (2005).
Reddy, L.R., Saravanan, P. & Corey, E.J. A simple stereocontrolled synthesis of salinosporamide A. J. Am. Chem. Soc. 126, 6230–6231 (2004).
Endo, A. & Danishefsky, S.J. Total synthesis of salinosporamide A. J. Am. Chem. Soc. 127, 8298–8299 (2005).
Mulholland, N.P., Pattenden, G. & Walters, I.A.S. A concise total synthesis of salinosporamide A. Org. Biomol. Chem. 4, 2845–2846 (2006).
Buchanan, G.O. et al. Sporolides A and B: structurally unprecedented halogenated macrolides from the marine actinomycete Salinispora tropica. Org. Lett. 7, 2731–2734 (2005).
Jensen, P.R. & Mafnas, C. Biogeography of the marine actinomycete Salinispora. Environ. Microbiol. 8, 1881–1888 (2006).
Kim, T.K., Hewavitharana, A.K., Shaw, P.N. & Fuerst, J.A. Discovery of a new source of rifamycin antibiotics in marine sponge actinobacteria by phylogenetic prediction. Appl. Environ. Microbiol. 72, 2118–2125 (2006).
Renner, M.K. et al. Cyclomarins A-C, New anti-inflammatory cyclic peptides produced by a marine bacterium (Streptomyces sp.). J. Am. Chem. Soc. 121, 11273–11276 (1999).
He, H. et al. Lomaiviticins A and B, potent antitumor antibiotics from Micromonospora lomaivitiensis. J. Am. Chem. Soc. 123, 5362–5363 (2001).
Charan, R.D. et al. Diazepinomicin, a new antimicrobial alkaloid from a marine Micromonospora sp. J. Nat. Prod. 67, 1431–1433 (2004).
Bernan, V.S., Greenstein, M. & Carter, G.T. Mining marine microorganisms as a source of new antimicrobials and antifungals. Curr. Med. Chem. Anti-infective Agents 3, 181–195 (2004).
Oh, D.C., Williams, P.G., Kauffman, C.A., Jensen, P.R. & Fenical, W. Cyanosporasides A and B, chloro- and cyano-cyclopenta[a]indene glycosides from the marine actinomycete “Salinispora pacifica”. Org. Lett. 8, 1021–1024 (2006).
Liu, W. et al. Rapid PCR amplification of minimal enediyne polyketide synthase cassettes leads to a predictive familial classification model. Proc. Natl. Acad. Sci. USA 100, 11959–11963 (2003).
Larsen, T.O., Smedsgaard, J., Nielsen, K.F., Hansen, M.E. & Frisvad, J.C. Phenotypic taxonomy and metabolite profiling in microbial drug discovery. Nat. Prod. Rep. 22, 672–695 (2005).
Ward, A.C. & Bora, N. Diversity and biogeography of marine actinobacteria. Curr. Opin. Microbiol. 9, 279–286 (2006).
Kwon, H.C., Kauffman, C.A., Jensen, P.R. & Fenical, W. Marinomycins A-D, antitumor antibiotics of a new structure class from a amrine actinomycete of the recently discovered genus “Marinispora”. J. Am. Chem. Soc. 128, 1622–1632 (2006).
Schlegel, R., Thrum, H., Zielinski, J. & Borowski, E.J. The structure of roflamycin, a new polyene macrolide antifungal antibiotic. J. Antibiot. (Tokyo) 34, 122–123 (1981).
Rychnovsky, S.D., Greisgraber, G. & Schlegel, R. Stereochemical determination of roflamycoin: 13C acetonide analysis and synthetic correlation. J. Am. Chem. Soc. 117, 197–210 (1995).
Neu, H.C. The crisis in antibiotic resistance. Science 257, 1064–1073 (1992).
Pathirana, C., Jensen, P.R. & Fenical, W. Marinone and debromomarinone, two phenylated napthoquinones from a marine actinomycete. Tetrahedron Lett. 33, 7663–7666 (1992).
Soria-Mercardo, I.E., Prieto-Davo, A., Jensen, P.R. & Fenical, W. Antibiotic terpenoid chloro-dihydroquinones from a new marine actinomycete. J. Nat. Prod. 68, 904–910 (2005).
Gribble, G.W. in The Handbook of Environmental Chemistry, Vol. 3, Part P, 1–15 (Springer-Verlag, Berlin, 2003).
Carter-Franklin, J.N., Parrish, J.D., Tschirret-Guth, R.A., Little, R.D. & Butler, A. Vanadium haloperoxidase-catalyzed bromination and cyclization of terpenes. J. Am. Chem. Soc. 125, 3688–3689 (2003).
Magarvey, N.A., Keller, J.M., Bernan, V., Dworkin, M. & Sherman, D.H. Isolation and characterization of novel marine-derived actinomycete taxa rich in bioactive metabolites. Appl. Environ. Microbiol. 70, 7520–7529 (2004).
Fiedler, H.-P. et al. Marine actinomycetes as a source of novel secondary metabolites. Antonie Van Leeuwenhoek 87, 37–42 (2005).
Bister, B. et al. Abyssomicin C - a polycyclic antibiotic from a marine Verrucosispora strain as an inhibitor of the para-aminobenzoic acid/tetrahydrofolate biosynthesis pathway. Angew. Chem. Int. Edn. Engl. 43, 2574–2576 (2004).
Newman, D.J. & Cragg, G.M. Marine natural products and related compounds in clinical and advanced preclinical trials. J. Nat. Prod. 67, 1216–1238 (2004).
Stach, J.E.M. et al. Statistical approaches for estimating actinobcterial diversity in marine sediments. Appl. Environ. Microbiol. 69, 6189–6200 (2003).
Naumann, K. Influence of chlorine substituents on biological activity of chemicals. J. Prakt. Chem. 341, 417–435 (1999).
Harris, C., Kannan, R., Kopecka, H. & Harris, T.M. The role of the chlorine substituents in the antibiotic vancomycin: preparation and characterization of mono- and didechlorovancomycin. J. Am. Chem. Soc. 107, 6652–6658 (1985).
Pfeifer, B.A. & Khosla, C. Biosynthesis of polyketides in heterologous hosts. Microbiol. Mol. Biol. Rev. 65, 106–118 (2001).
Walsh, C.T. Combinatorial biosynthesis of antibiotics: challenges and opportunities. ChemBioChem 3, 125–134 (2002).
Janssen, P.H., Yates, P.S., Grinton, B.E., Taylor, P.M. & Sait, M. Improved culturability of soil bacteria and isolation in pure culture of novel members of the Divisions Acidobacteria, Actinobacteria, Proteobacteria, and Verrucomicrobia. Appl. Environ. Microbiol. 68, 2391–2396 (2002).
Challis, G.L. & Ravel, J. Coelichen, a new peptide siderophore encoded by the Streptomyces coelicolor genome: structure prediction from the sequence of its non-ribosomal peptide synthetase. FEMS Microbiol. Lett. 187, 111–114 (2000).
Acknowledgements
We thank the US National Institutes of Health, National Cancer Institute for generous continuing support of this research under grants CA44848, CA052955 and CA048112. Support from the NCI allowed the unusual marine microbiology described herein to be explored. We thank V. Bernan (Wyeth Research Laboratories) for S. pacifica sequence data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
W.F. and P.J. are founders of and consultants to Nereus Pharmaceuticals.
Rights and permissions
About this article
Cite this article
Fenical, W., Jensen, P. Developing a new resource for drug discovery: marine actinomycete bacteria. Nat Chem Biol 2, 666–673 (2006). https://doi.org/10.1038/nchembio841
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio841
This article is cited by
-
Biogeographic patterns of biosynthetic potential and specialized metabolites in marine sediments
The ISME Journal (2023)
-
1-(6-Methylsalicyloyl)glycerol from stony coral-derived Micromonospora sp.
The Journal of Antibiotics (2023)
-
Novel Bioactive Compounds from Streptomyces sp. NLKPB45 Isolated from Marine Soil Sediment
Pharmaceutical Chemistry Journal (2023)
-
Uncovering the biodiversity and biosynthetic potentials of rare actinomycetes
Future Journal of Pharmaceutical Sciences (2022)
-
Diversity and antimicrobial activities of culturable actinomycetes from Odontotermes formosanus (Blattaria: Termitidae)
BMC Microbiology (2022)