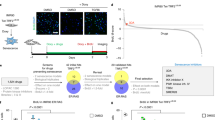
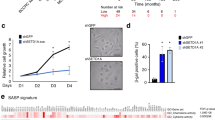
Abstract
Most somatic cells encounter an inevitable destiny, senescence1,2. Little progress has been made in identifying small molecules that extend the finite lifespan of normal human cells. Here we show that the intrinsic 'senescence clock' can be reset in a reversible manner by selective modulation of the ataxia telangiectasia–mutated (ATM) protein and ATM- and Rad3-related (ATR) protein with a small molecule, CGK733. This compound was identified by a high-throughput phenotypic screen with automated imaging. Employing a magnetic nanoprobe technology, magnetism-based interaction capture (MAGIC)3, we identified ATM as the molecular target of CGK733 from a genome-wide screen. CGK733 inhibits ATM and ATR kinase activities and blocks their checkpoint signaling pathways with great selectivity. Consistently, siRNA-mediated knockdown of ATM and ATR induced the proliferation of senescent cells, although with lesser efficiency than CGK733. These results might reflect the specific targeting of the kinase activities of ATM and ATR by CGK733 without affecting any other domains required for cell proliferation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
22 December 2006
In the version of this article initially published, no competing financial interests were declared. The authors now declare that they have competing interests that might be perceived to influence the results and discussion reported in this paper, which are detailed in a declaration of competing financial interests accompanying the article. The error has been corrected in the HTML and PDF versions of the article.
References
Wright, W.E. & Shay, J.W. Historical claims and current interpretations of replicative aging. Nat. Biotechnol. 20, 682–688 (2002).
Campisi, J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120, 513–522 (2005).
Won, J. et al. A magnetic nanoprobe technology for detecting molecular interactions in live cells. Science 309, 121–125 (2005).
Jacobs, J.J. et al. Senescence bypass screen identifies TBX2, which represses Cdkn2a (p19ARF) and is amplified in a subset of human breast cancers. Nat. Genet. 26, 291–299 (2000).
Gil, J., Bernard, D., Martinez, D. & Beach, D. Polycomb CBX7 has a unifying role in cellular lifespan. Nat. Cell Biol. 6, 67–72 (2004).
Bodnar, A.G. et al. Extension of life-span by introduction of telomerase into normal human cells. Science 279, 349–352 (1998).
Crews, C.M. & Splittgerber, U. Chemical genetics: exploring and controlling cellular processes with chemical probes. Trends Biochem. Sci. 24, 317–320 (1999).
Schreiber, S.L. Small molecules: the missing link in the central dogma. Nat. Chem. Biol. 1, 64–66 (2005).
van Steensel, B., Smogorzewska, A. & de Lange, T. TRF2 protects human telomeres from end-to-end fusions. Cell 92, 401–413 (1998).
Takai, H., Smogorzewska, A. & de Lange, T. DNA damage foci at dysfunctional telomeres. Curr. Biol. 13, 1549–1556 (2003).
Stockwell, B.R. Exploring biology with small organic molecules. Nature 432, 846–854 (2004).
Burdine, L. & Kodadek, T. Target identification in chemical genetics: the (often) missing link. Chem. Biol. 11, 593–597 (2004).
Cohen, P. Protein kinases—the major drug targets of the twenty-first century? Nat. Rev. Drug Discov. 1, 309–315 (2002).
Shiloh, Y. ATM and related protein kinases: safeguarding genome integrity. Nat. Rev. Cancer 3, 155–168 (2003).
Kastan, M.B. & Bartek, J. Cell cycle checkpoints and cancer. Nature 432, 316–323 (2004).
Bode, A.M. & Dong, Z. Post-translational modification of p53 in tumorigenesis. Nat. Rev. Cancer 4, 793–805 (2004).
Zimber, A., Nguyen, Q.D. & Gespach, C. Nuclear bodies and compartments: functional roles and cellular signalling in health and disease. Cell. Signal. 16, 1085–1104 (2004).
Vivanco, I. & Sawyers, C.L. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat. Rev. Cancer 2, 489–501 (2002).
Tresini, M., Mawal-dewan, M., Cristofalo, V.J. & Sell, C. A phosphatidylinositol 3-kinase inhibitor induces a senescent-like growth arrest in human diploid fibroblast cells. Cancer Res. 58, 1–4 (1998).
Sarbassov, D.D., Guertin, D.A., Ali, S.M. & Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the Rictor-mTOR complex. Science 307, 1098–1101 (2005).
Herbig, U., Jobling, W., Chen, B.P.C., Chen, D.J. & Sedivy, J.M. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21CIP1, but not p16INK4a. Mol. Cell 14, 501–513 (2004).
Ben-Porath, I. & Weinberg, R.A. The signals and pathways activating cellular senescence. Int. J. Biochem. Cell Biol. 37, 961–976 (2005).
Karlseder, J., Broccoli, D., Dai, Y., Hardy, S. & de Lange, T. p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science 283, 1321–1325 (1999).
d'Adda di Fagagna, F. et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature 426, 194–198 (2003).
Hickson, I. et al. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 64, 9152–9159 (2004).
Pandita, T.K. ATM function and telomere stability. Oncogene 21, 611–618 (2002).
Ito, K. et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature 431, 997–1002 (2004).
Brown, E.J. & Baltimore, D. Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance. Genes Dev. 17, 615–628 (2003).
Shechter, D., Costanzo, V. & Gautier, J. ATR and ATM regulate the timing of DNA replication origin firing. Nat. Cell Biol. 6, 648–655 (2004).
Dimri, G.P. et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 92, 9363–9367 (1995).
Acknowledgements
We thank T. de Lange, R.Y. Tsien, J. Campisi, J. Chung, G.P. Nolan, M.R. Stampfer and D.S. Lim for gifts of reagents. This work was supported by CGK Co. Ltd. and was also partially supported by the Korea Research Foundation grant (KRF-2005-C00097), the Korea Health 21 R&D Project (A040042) and the Chemical Genomics program from the Korean Ministry of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
T.K.K. receives support for research programs from CGK Co., Ltd. M.K. has a part-time consulting relationship with CGK Co., Ltd.*
*Note: In the version of this article initially published, no competing financial interests were declared. The authors now declare that they have competing interests that might be perceived to influence the results and discussion reported in this paper, which are detailed in a declaration of competing financial interests accompanying the article. The error has been corrected in the HTML and PDF versions of the article.
Supplementary information
Supplementary Fig. 1
Establishment of cellular senescence by overexpression of TRF2ΔBΔM. (PDF 35 kb)
Supplementary Fig. 2
Reversal of replicative senescence of BJ and HMEC cells by CGK733. (PDF 160 kb)
Supplementary Fig. 3
Karyotypes of the senescent cells induced to proliferate by CGK733. (PDF 7 kb)
Supplementary Fig. 4
Effects of CGK733 on ATM and ATR kinase activities inside cells. (PDF 63 kb)
Supplementary Fig. 5
Effects of ATM/ATR siRNAs and CGK733 on the proliferation of senescent cells. (PDF 8 kb)
Supplementary Fig. 6
Synthesis of KU-55933 and a CGK733-biotin derivative. (PDF 26 kb)
Supplementary Fig. 7
Effects of KU-55933 on replicative senescence. (PDF 7 kb)
Rights and permissions
About this article
Cite this article
Won, J., Kim, M., Kim, N. et al. Small molecule–based reversible reprogramming of cellular lifespan. Nat Chem Biol 2, 369–374 (2006). https://doi.org/10.1038/nchembio800
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio800
This article is cited by
-
H2AX phosphorylation and DNA damage kinase activity are dispensable for herpes simplex virus replication
Virology Journal (2016)
-
The ATM and ATR inhibitors CGK733 and caffeine suppress cyclin D1 levels and inhibit cell proliferation
Radiation Oncology (2009)
-
Retraction Note: Small molecule–based reversible reprogramming of cellular lifespan
Nature Chemical Biology (2008)
-
Correcting the scientific record
Nature Chemical Biology (2008)
-
Corrigendum: Small molecule–based reversible reprogramming of cellular lifespan
Nature Chemical Biology (2007)