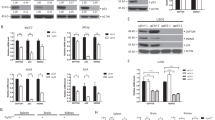
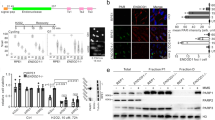
Abstract
The p53 tumor suppressor orchestrates alternative stress responses including cell cycle arrest and apoptosis, but the mechanisms defining cell fate upon p53 activation are poorly understood. Several small-molecule activators of p53 have been developed, including Nutlin-3, but their therapeutic potential is limited by the fact that they induce reversible cell cycle arrest in most cancer cell types. We report here the results of a genome-wide short hairpin RNA screen for genes that are lethal in combination with p53 activation by Nutlin-3, which showed that the ATM and MET kinases govern cell fate choice upon p53 activation. Genetic or pharmacological interference with ATM or MET activity converts the cellular response from cell cycle arrest into apoptosis in diverse cancer cell types without affecting expression of key p53 target genes. ATM and MET inhibitors also enable Nutlin-3 to kill tumor spheroids. These results identify new pathways controlling the cellular response to p53 activation and aid in the design of p53-based therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Vousden, K.H. & Prives, C. Blinded by the light: the growing complexity of p53. Cell 137, 413–431 (2009).
Brown, C.J., Lain, S., Verma, C.S., Fersht, A.R. & Lane, D.P. Awakening guardian angels: drugging the p53 pathway. Nat. Rev. Cancer 9, 862–873 (2009).
Levesque, A.A. & Eastman, A. p53-based cancer therapies: is defective p53 the Achilles heel of the tumor? Carcinogenesis 28, 13–20 (2007).
Mandinova, A. & Lee, S.W. The p53 pathway as a target in cancer therapeutics: obstacles and promise. Sci. Transl. Med. 3, 64rv1 (2011).
Vassilev, L.T. et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303, 844–848 (2004).
Tovar, C. et al. Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy. Proc. Natl. Acad. Sci. USA 103, 1888–1893 (2006).
Huang, B., Deo, D., Xia, M. & Vassilev, L.T. Pharmacologic p53 activation blocks cell cycle progression but fails to induce senescence in epithelial cancer cells. Mol. Cancer Res. 7, 1497–1509 (2009).
París, R., Henry, R.E., Stephens, S.J., McBryde, M. & Espinosa, J.M. Multiple p53-independent gene silencing mechanisms define the cellular response to p53 activation. Cell Cycle 7, 2427–2433 (2008).
Ventura, A. et al. Restoration of p53 function leads to tumour regression in vivo. Nature 445, 661–665 (2007).
Xue, W. et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660 (2007).
el-Deiry, W.S. et al. WAF1, a potential mediator of p53 tumor suppression. Cell 75, 817–825 (1993).
Hermeking, H. et al. 14–3-3σ is a p53-regulated inhibitor of G2/M progression. Mol. Cell 1, 3–11 (1997).
Yu, J., Zhang, L., Hwang, P.M., Kinzler, K.W. & Vogelstein, B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol. Cell 7, 673–682 (2001).
Nakano, K. & Vousden, K.H. PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell 7, 683–694 (2001).
Wu, G.S. et al. KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat. Genet. 17, 141–143 (1997).
Müller, M. et al. p53 activates the CD95 (APO-1/Fas) gene in response to DNA damage by anticancer drugs. J. Exp. Med. 188, 2033–2045 (1998).
Vousden, K.H. & Lu, X. Live or let die: the cell's response to p53. Nat. Rev. Cancer 2, 594–604 (2002).
Sullivan, K.D., Gallant-Behm, C.L., Henry, R.E., Fraikin, J.L. & Espinosa, J.M. The p53 circuit board. Biochim. Biophys. Acta 1825, 229–244 (2012).
Bunz, F. et al. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J. Clin. Invest. 104, 263–269 (1999).
Kim, J. & Tan, A.C. BiNGS!SL-seq: a bioinformatics pipeline for the analysis and interpretation of deep sequencing genome-wide synthetic lethal screen. Methods Mol. Biol. 802, 389–398 (2012).
Porter, C.C. et al. Integrated genomic analyses identify WEE1 as a critical mediator of cell fate and a novel therapeutic target in acute myeloid leukemia. Leukemia published online; doi:10.1038/leu.2011.392 (13 January 2012).
Di Renzo, M.F. et al. Overexpression and amplification of the met/HGF receptor gene during the progression of colorectal cancer. Clin. Cancer Res. 1, 147–154 (1995).
Takhar, A.S., Eremin, O. & Watson, S.A. The role of gastrin in colorectal carcinogenesis. Surgeon 2, 251–257 (2004).
Li, A., Varney, M.L. & Singh, R.K. Expression of interleukin 8 and its receptors in human colon carcinoma cells with different metastatic potentials. Clin. Cancer Res. 7, 3298–3304 (2001).
Sugai, T. et al. Frequent allelic imbalance at the ATM locus in DNA multiploid colorectal carcinomas. Oncogene 20, 6095–6101 (2001).
Hickson, I. et al. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 64, 9152–9159 (2004).
Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 70, 440–446 (2010).
Friedrich, J., Seidel, C., Ebner, R. & Kunz-Schughart, L.A. Spheroid-based drug screen: considerations and practical approach. Nat. Protoc. 4, 309–324 (2009).
Olivero, M. et al. Overexpression and activation of hepatocyte growth factor/scatter factor in human non-small-cell lung carcinomas. Br. J. Cancer 74, 1862–1868 (1996).
Yamazaki, S. et al. Pharmacokinetic-pharmacodynamic modeling of biomarker response and tumor growth inhibition to an orally available cMet kinase inhibitor in human tumor xenograft mouse models. Drug Metab. Dispos. 36, 1267–1274 (2008).
Shaw, A.T. & Solomon, B. Targeting anaplastic lymphoma kinase in lung cancer. Clin. Cancer Res. 17, 2081–2086 (2011).
Sattler, M. et al. A novel small molecule met inhibitor induces apoptosis in cells transformed by the oncogenic TPR-MET tyrosine kinase. Cancer Res. 63, 5462–5469 (2003).
Hwang, C.I. et al. Wild-type p53 controls cell motility and invasion by dual regulation of MET expression. Proc. Natl. Acad. Sci. USA 108, 14240–14245 (2011).
Zhang, Y., Xing, D. & Liu, L. PUMA promotes Bax translocation by both directly interacting with Bax and by competitive binding to Bcl-X L during UV-induced apoptosis. Mol. Biol. Cell 20, 3077–3087 (2009).
Chan, T.A., Hwang, P.M., Hermeking, H., Kinzler, K.W. & Vogelstein, B. Cooperative effects of genes controlling the G(2)/M checkpoint. Genes Dev. 14, 1584–1588 (2000).
Henry, R.E., Andrysik, Z., Paris, R., Galbraith, M.D. & Espinosa, J.M.A. DR4:tBID axis drives the p53 apoptotic response by promoting oligomerization of poised BAX. EMBO J. 31, 1266–1278 (2012).
Jiang, H. et al. The combined status of ATM and p53 link tumor development with therapeutic response. Genes Dev. 23, 1895–1909 (2009).
Brummelkamp, T.R. et al. An shRNA barcode screen provides insight into cancer cell vulnerability to MDM2 inhibitors. Nat. Chem. Biol. 2, 202–206 (2006).
Biton, S. & Ashkenazi, A. NEMO and RIP1 control cell fate in response to extensive DNA damage via TNF-α feedforward signaling. Cell 145, 92–103 (2011).
Guo, Z., Kozlov, S., Lavin, M.F., Person, M.D. & Paull, T.T. ATM activation by oxidative stress. Science 330, 517–521 (2010).
Hadian, K. & Krappmann, D. Signals from the nucleus: activation of NF-κB by cytosolic ATM in the DNA damage response. Sci. Signal. 4, pe2 (2011).
Bykov, V.J. et al. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat. Med. 8, 282–288 (2002).
Issaeva, N. et al. Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nat. Med. 10, 1321–1328 (2004).
Lavin, M.F. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat. Rev. Mol. Cell Biol. 9, 759–769 (2008).
Miyamoto, S. Nuclear initiated NF-κB signaling: NEMO and ATM take center stage. Cell Res. 21, 116–130 (2011).
Ding, J., Miao, Z.H., Meng, L.H. & Geng, M.Y. Emerging cancer therapeutic opportunities target DNA-repair systems. Trends Pharmacol. Sci. 27, 338–344 (2006).
Trusolino, L., Bertotti, A. & Comoglio, P.M. MET signalling: principles and functions in development, organ regeneration and cancer. Nat. Rev. Mol. Cell Biol. 11, 834–848 (2010).
Soda, M. et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 448, 561–566 (2007).
Kwak, E.L. et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N. Engl. J. Med. 363, 1693–1703 (2010).
Acknowledgements
This work was supported by US National Institutes of Health grant RO1 CA117907, a Lung SPORE Pilot Grant (P50 CA058187), a pilot grant from the Cancer League of Colorado and a Career Development Award from The Leukemia and Lymphoma Society to K.D.S. J.M.E. is a Howard Hughes Medical Institute Early Career Scientist. We thank members of the Espinosa lab for support and discussions and H. Kennedy and J. Kruk for inspiration.
Author information
Authors and Affiliations
Contributions
K.D.S. conducted most experiments, interpreted all data and wrote the paper. N.P.-J. did cell culture, western blots and cell viability assays. R.E.H. carried out the microarray experiment. C.C.P. and J.D. shared unpublished protocols for synthetic lethal screens in human cells. J.K. and A.C.T. developed BiNGS and did most bioinformatics analyses. J.J.T. and S.G.E. conducted MCTS experiments and analyzed CI data. J.M.E. participated in project design, established the collaborations and co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results (PDF 5250 kb)
Supplementary Data Set 1
HCT116 screen data (XLSX 1642 kb)
Supplementary Data Set 2
Raw sequence counts from HCT116 screen (XLSX 2403 kb)
Supplementary Data Set 3
A549 screen data (XLSX 1204 kb)
Supplementary Data Set 4
Raw sequence counts from A549 screen (XLSX 3443 kb)
Supplementary Data Set 5
shRNA sequences used in this study (XLSX 58 kb)
Rights and permissions
About this article
Cite this article
Sullivan, K., Padilla-Just, N., Henry, R. et al. ATM and MET kinases are synthetic lethal with nongenotoxic activation of p53. Nat Chem Biol 8, 646–654 (2012). https://doi.org/10.1038/nchembio.965
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.965
This article is cited by
-
PPM1D suppresses p53-dependent transactivation and cell death by inhibiting the Integrated Stress Response
Nature Communications (2022)
-
Resistance mechanisms to inhibitors of p53-MDM2 interactions in cancer therapy: can we overcome them?
Cellular & Molecular Biology Letters (2021)
-
p53 induces formation of NEAT1 lncRNA-containing paraspeckles that modulate replication stress response and chemosensitivity
Nature Medicine (2016)
-
p53 in survival, death and metabolic health: a lifeguard with a licence to kill
Nature Reviews Molecular Cell Biology (2015)
-
ATM signalling and cancer
Oncogene (2014)