Abstract
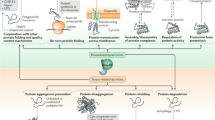
Protein homeostasis (proteostasis) is essential for cellular and organismal health. Stress, aging and the chronic expression of misfolded proteins, however, challenge the proteostasis machinery and the vitality of the cell. Enhanced expression of molecular chaperones, regulated by heat shock transcription factor-1 (HSF-1), has been shown to restore proteostasis in a variety of conformational disease models, suggesting this mechanism as a promising therapeutic approach. We describe the results of a screen comprised of ∼900,000 small molecules that identified new classes of small-molecule proteostasis regulators that induce HSF-1–dependent chaperone expression and restore protein folding in multiple conformational disease models. These beneficial effects to proteome stability are mediated by HSF-1, FOXO, Nrf-2 and the chaperone machinery through mechanisms that are distinct from current known small-molecule activators of the heat shock response. We suggest that modulation of the proteostasis network by proteostasis regulators may be a promising therapeutic approach for the treatment of a variety of protein conformational diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Balch, W.E., Morimoto, R.I., Dillin, A. & Kelly, J.W. Adapting proteostasis for disease intervention. Science 319, 916–919 (2008).
Powers, E.T., Morimoto, R.I., Dillin, A., Kelly, J.W. & Balch, W.E. Biological and chemical approaches to diseases of proteostasis deficiency. Annu. Rev. Biochem. 78, 959–991 (2009).
Morimoto, R.I. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 22, 1427–1438 (2008).
Ron, D. & Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529 (2007).
Haynes, C.M. & Ron, D. The mitochondrial UPR - protecting organelle protein homeostasis. J. Cell Sci. 123, 3849–3855 (2010).
Lichtlen, P. & Schaffner, W. Putting its fingers on stressful situations: the heavy metal-regulatory transcription factor MTF-1. Bioessays 23, 1010–1017 (2001).
Vargas, M.R. & Johnson, J.A. The Nrf2-ARE cytoprotective pathway in astrocytes. Expert Rev. Mol. Med. 11, e17 (2009).
Åkerfelt, M., Morimoto, R.I. & Sistonen, L. Heat shock factors: integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 11, 545–555 (2010).
Young, J.C., Agashe, V.R., Siegers, K. & Hartl, F.U. Pathways of chaperone-mediated protein folding in the cytosol. Nat. Rev. Mol. Cell Biol. 5, 781–791 (2004).
Gidalevitz, T., Ben-Zvi, A., Ho, K.H., Brignull, H.R. & Morimoto, R.I. Progressive disruption of cellular protein folding in models of polyglutamine diseases. Science 311, 1471–1474 (2006).
Gidalevitz, T., Kikis, E.A. & Morimoto, R.I. A cellular perspective on conformational disease: the role of genetic background and proteostasis networks. Curr. Opin. Struct. Biol. 20, 23–32 (2010).
Sittler, A. et al. Geldanamycin activates a heat shock response and inhibits huntingtin aggregation in a cell culture model of Huntington′s disease. Hum. Mol. Genet. 10, 1307–1315 (2001).
Muchowski, P.J. & Wacker, J.L. Modulation of neurodegeneration by molecular chaperones. Nat. Rev. Neurosci. 6, 11–22 (2005).
Fujimoto, M. et al. Active HSF1 significantly suppresses polyglutamine aggregate formation in cellular and mouse models. J. Biol. Chem. 280, 34908–34916 (2005).
Vacher, C., Garcia-Oroz, L. & Rubinsztein, D.C. Overexpression of yeast hsp104 reduces polyglutamine aggregation and prolongs survival of a transgenic mouse model of Huntington's disease. Hum. Mol. Genet. 14, 3425–3433 (2005).
Waza, M. et al. 17-AAG, an Hsp90 inhibitor, ameliorates polyglutamine-mediated motor neuron degeneration. Nat. Med. 11, 1088–1095 (2005).
Zhang, Y.Q. & Sarge, K.D. Celastrol inhibits polyglutamine aggregation and toxicity though induction of the heat shock response. J. Mol. Med. 85, 1421–1428 (2007).
Fujikake, N. et al. Heat shock transcription factor 1–activating compounds suppress polyglutamine-induced neurodegeneration through induction of multiple molecular chaperones. J. Biol. Chem. 283, 26188–26197 (2008).
Hsu, A.L., Murphy, C.T. & Kenyon, C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 300, 1142–1145 (2003).
Neef, D.W., Turski, M.L. & Thiele, D.J. Modulation of heat shock transcription factor 1 as a therapeutic target for small molecule intervention in neurodegenerative disease. PLoS Biol. 8, e1000291 (2010).
Westerheide, S.D. & Morimoto, R.I. Heat shock response modulators as therapeutic tools for diseases of protein conformation. J. Biol. Chem. 280, 33097–33100 (2005).
Taldone, T., Gozman, A., Maharaj, R. & Chiosis, G. Targeting Hsp90: small-molecule inhibitors and their clinical development. Curr. Opin. Pharmacol. 8, 370–374 (2008).
Luo, W., Sun, W., Taldone, T., Rodina, A. & Chiosis, G. Heat shock protein 90 in neurodegenerative diseases. Mol. Neurodegener. 5, 24 (2010).
Solit, D.B. et al. Phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with metastatic melanoma. Clin. Cancer Res. 14, 8302–8307 (2008).
Westerheide, S.D. et al. Celastrols as inducers of the heat shock response and cytoprotection. J. Biol. Chem. 279, 56053–56060 (2004).
Williams, G.T. & Morimoto, R.I. Maximal stress-induced transcription from the human HSP70 promoter requires interactions with the basal promoter elements independent of rotational alignment. Mol. Cell. Biol. 10, 3125–3136 (1990).
Butina, D. Unsupervised data base clustering based on Daylight′s fingerprint and Tanimoto similarity: a fast and automated way to cluster small and large data sets. J. Chem. Inf. Comput. Sci. 39, 747–750 (1999).
Amici, C., Sistonen, L., Santoro, M.G. & Morimoto, R.I. Antiproliferative prostaglandins activate heat shock transcription factor. Proc. Natl. Acad. Sci. USA 89, 6227–6231 (1992).
Trott, A. et al. Activation of heat shock and antioxidant responses by the natural product celastrol: transcriptional signatures of a thiol-targeted molecule. Mol. Biol. Cell 19, 1104–1112 (2008).
Li, C. et al. Pharmacologic induction of heme oxygenase-1. Antioxid. Redox Signal. 9, 2227–2239 (2007).
Liu, Y. & Chang, A. Heat shock response relieves ER stress. EMBO J. 27, 1049–1059 (2008).
Wyttenbach, A. et al. Polyglutamine expansions cause decreased CRE-mediated transcription and early gene expression changes prior to cell death in an inducible cell model of Huntington′s disease. Hum. Mol. Genet. 10, 1829–1845 (2001).
Riordan, J.R. CFTR function and prospects for therapy. Annu. Rev. Biochem. 77, 701–726 (2008).
Morley, J.F., Brignull, H.R., Weyers, J.J. & Morimoto, R.I. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 99, 10417–10422 (2002).
Nollen, E.A. et al. Genome-wide RNA interference screen identifies previously undescribed regulators of polyglutamine aggregation. Proc. Natl. Acad. Sci. USA 101, 6403–6408 (2004).
Garcia, S.M., Casanueva, M.O., Silva, M.C., Amaral, M.D. & Morimoto, R.I. Neuronal signaling modulates protein homeostasis in Caenorhabditis elegans post-synaptic muscle cells. Genes Dev. 21, 3006–3016 (2007).
Whitesell, L. & Lindquist, S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 5, 761–772 (2005).
Stringer, J.R., Bowman, M.D., Weisblum, B. & Blackwell, H.E. Improved small-molecule macroarray platform for the rapid synthesis and discovery of antibacterial chalcones. ACS Comb. Sci. 13, 175–180 (2011).
Yadav, V.R., Prasad, S., Sung, B. & Aggarwal, B.B. The role of chalcones in suppression of NF-κB–mediated inflammation and cancer. Int. Immunopharmacol. 11, 295–309 (2010).
Dinkova-Kostova, A.T., Massiah, M.A., Bozak, R.E., Hicks, R.J. & Talalay, P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc. Natl. Acad. Sci. USA 98, 3404–3409 (2001).
Dinkova-Kostova, A.T. et al. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA 99, 11908–11913 (2002).
Tangallapally, R.P. et al. Synthesis and evaluation of nitrofuranylamides as novel antituberculosis agents. J. Med. Chem. 47, 5276–5283 (2004).
Roesslein, M. et al. Thiopental protects human T lymphocytes from apoptosis in vitro via the expression of heat shock protein 70. J. Pharmacol. Exp. Ther. 325, 217–225 (2008).
Yang, H. et al. Nanomolar affinity small molecule correctors of defective Δ F508-CFTR chloride channel gating. J. Biol. Chem. 278, 35079–35085 (2003).
Yang, F. et al. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 280, 5892–5901 (2005).
Teiten, M.H., Reuter, S., Schmucker, S., Dicato, M. & Diederich, M. Induction of heat shock response by curcumin in human leukemia cells. Cancer Lett. 279, 145–154 (2009).
Mu, T.W. et al. Chemical and biological approaches synergize to ameliorate protein-folding diseases. Cell 134, 769–781 (2008).
Parenti, G. Treating lysosomal storage diseases with pharmacological chaperones: from concept to clinics. EMBO Mol. Med. 1, 268–279 (2009).
Gorbatyuk, M.S. et al. Restoration of visual function in P23H rhodopsin transgenic rats by gene delivery of BiP/Grp78. Proc. Natl. Acad. Sci. USA 107, 5961–5966 (2010).
Tam, L.C. et al. Prevention of autosomal dominant retinitis pigmentosa by systemic drug therapy targeting heat shock protein 90 (Hsp90). Hum. Mol. Genet. 19, 4421–4436 (2010).
Acknowledgements
We acknowledge J. Maddry, the SRI and the NINDS for support in performing the primary screen; P. Chase and P. Baillargeon of Scripps Florida for executing the SRIMSC screening activities; S. Fox, J. West, S. Westerheide, J. Moran, M. Beam and K. Orton for technical assistance; J.S. Pedersen for help developing the worm tracker system; and the Morimoto laboratory, in particular T. Gidalevitz, J. Kirstein, C. Voisine and A. Yu, for helpful comments. PC12 httQ74-GFP cells were provided by D. Rubinsztein (University of Cambridge). Purified Hsp90β was a gift of A. Chadli (Georgia Health Science University). We thank A. Ciechanover for the rabbit polyclonal ubiquitin antibody (Israel Institute of Technology). The CFBE41o- YFP cells were a gift from L. Galietta (Istituto Giannina Gaslini). This work was supported by the US National Institutes of Health (NIH) Training Grant in Signal Transduction and Cancer T32 CA70085 and the NIH Training Grant in Drug Discovery in Age Related Diseases T32 AG000260 (to B.C.), Portuguese PhD fellowship from the Fundação para a Ciência e Tecnologia (SFRH/BD/28461/2006) (to M.C.S.), NIH grants HL 079442, GM42336 and DK785483 (to W.E.B.), a fellowship from the Canadian Institutes for Health Research (CIHR) (to D.M.H.), the NIH Molecular Library Screening Center Network MH084512 (to F.M., S.A.S. and P.H.) and NIH grants GM038109, GM081192, AG026647 and NS047331 (to R.I.M.).
Author information
Authors and Affiliations
Contributions
B.C. and R.I.M. designed the research plan. B.C., M.C.S., F.M., M.A.C., D.M.H., S.K. and S.A.S. designed and performed the research. B.C., M.C.S., F.M., D.M.H., S.A.S., P.H., B.D.T., D.G., W.E.B. and R.I.M. analyzed and interpreted the data. B.C. and R.I.M. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
B.D.T., D.G. and S.K. are employees of Proteostasis Therapeutics Inc. (Cambridge, MA) that is developing small molecule therapeutics for protein misfolding diseases. W.E.B. is a co-founder, shareholder and a paid consultant for Proteostasis Therapeutics Inc. R.I.M. is founder, shareholder, and paid consultant for Proteostasis Therapeutics Inc.
Supplementary information
Supplementary Text and Figures
Supplementary Scheme 1, Supplementary Figures 1–13, Supplementary Tables 1–3 and Supplementary Methods (PDF 6321 kb)
Rights and permissions
About this article
Cite this article
Calamini, B., Silva, M., Madoux, F. et al. Small-molecule proteostasis regulators for protein conformational diseases. Nat Chem Biol 8, 185–196 (2012). https://doi.org/10.1038/nchembio.763
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.763
This article is cited by
-
Rapid differentiation of hiPSCs into functional oligodendrocytes using an OLIG2 synthetic modified messenger RNA
Communications Biology (2022)
-
Inhibition of the ubiquitin-proteasome system by an NQO1-activatable compound
Cell Death & Disease (2021)
-
Two human metabolites rescue a C. elegans model of Alzheimer’s disease via a cytosolic unfolded protein response
Communications Biology (2021)
-
Pharmacologic IRE1/XBP1s activation confers targeted ER proteostasis reprogramming
Nature Chemical Biology (2020)
-
Structure-guided combination therapy to potently improve the function of mutant CFTRs
Nature Medicine (2018)