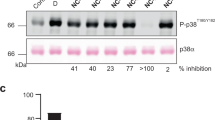
Abstract
Until now, a lack of inhibitors with high potency and selectivity in vivo has hampered investigation of the p38 mitogen-activated protein kinase (MAPK) signaling pathway. We describe the design of skepinone-L, which is, to our knowledge, the first ATP-competitive p38 MAPK inhibitor with excellent in vivo efficacy and selectivity. Therefore, skepinone-L is a valuable probe for chemical biology research, and it may foster the development of a unique class of kinase inhibitors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Wagner, E.F. & Nebreda, A.R. Nat. Rev. Cancer 9, 537–549 (2009).
Veillat, V. et al. J. Clin. Endocrinol. Metab. 95, E403–E412 (2010).
Xie, N. et al. Neuropharmacology 59, 444–451 (2010).
Goldstein, D.M. & Gabriel, T. Curr. Top. Med. Chem. 5, 1017–1029 (2005).
Karaman, M.W. et al. Nat. Biotechnol. 26, 127–132 (2008).
Lee, M.R. & Dominguez, C. Curr. Med. Chem. 12, 2979–2994 (2005).
Karcher, S.C. & Laufer, S.A. Curr. Top. Med. Chem. 9, 655–676 (2009).
Fitzgerald, C.E. et al. Nat. Struct. Biol. 10, 764–769 (2003).
Laufer, S.A., Ahrens, G.M., Karcher, S.C., Hering, J.S. & Niess, R. J. Med. Chem. 49, 7912–7915 (2006).
Karcher, S.C. & Laufer, S.A. J. Med. Chem. 52, 1778–1782 (2009).
Goldstein, D.M. et al. J. Med. Chem. 49, 1562–1575 (2006).
Dinarello, C.A. Semin. Dial. 22, 256–259 (2009).
Mutou, Y., Tsukimoto, M., Homma, T. & Kojima, S. J. Health Sci. 56, 675–683 (2010).
Schett, G., Zwerina, J. & Firestein, G. Ann. Rheum. Dis. 67, 909–916 (2008).
Ip, W.K., Wong, C.K. & Lam, C.W. Clin. Exp. Immunol. 145, 162–172 (2006).
Pan, S.L. et al. Shock 30, 496–502 (2008).
Peng, H. et al. J. Biol. Chem. 286, 24508–24518 (2011).
Schieven, G.L. Curr. Top. Med. Chem. 9, 1038–1048 (2009).
Shimada, H. & Rajagopalan, L.E. J. Biol. Chem. 285, 12536–12542 (2010).
Ip, W.K., Wong, C.K., Wang, C.B., Tian, Y.P. & Lam, C.W. Immunopharmacol. Immunotoxicol. 27, 371–393 (2005).
Tibbles, L.A. et al. J. Virol. 76, 1559–1568 (2002).
Cekic, C. et al. J. Biol. Chem. 284, 31982–31991 (2009).
Hill, R.J. et al. J. Pharmacol. Exp. Ther. 327, 610–619 (2008).
Kurihara, Y., Nakahara, T. & Furue, M. Cell. Immunol. 270, 25–31 (2011).
Manning, G., Whyte, D.B., Martinez, R., Hunter, T. & Sudarsanam, S. Science 6, 1912–1934 (2002).
Acknowledgements
We thank S. Luik, M Goettert and K. Bauer for biological testing. We thank M. Fecker, J.R. Simard, S. Mayer-Wrangowski and A. Richters for expert assistance during protein production and crystallization. D.R. is grateful for funds from the German Federal Ministry for Education (grant no. BMBF 01GS08104). We thank T. Joos, N. Schneiderhan-Marra, K. Hefner, M. Schmohl and G.M. Stein (Naturwissenschaftliches und Medizinisches Institut and Experimentelle und Diagnostische Immunologie Reutlingen) for cytokine profiling of skepinone-L in hPMBCs.
Author information
Authors and Affiliations
Contributions
S.A.L. and T.S. initiated and supervised this study, S.C.K. conceived the chemical experiments, and S.C.K. and S.F. carried them out, J.R. conceived the experiment for X-ray structure of 2-amino-phenylamino-dibenzosuberone and carried it out. V.S. performed Molecular Modeling. C.G. solved the complex crystal structure of skepinone-L and carried out HeLa cell experiments with skepinone-L. A.K. conceived the cellular selectivity experiments and A.K and S.C.K. carried them out; W.A. conceived and carried out the studies for in vivo experiments. S.C.K., A.K., J.R., C.G. and W.A. designed and carried out the data analysis. S.C.K., A.K., S.F., D.R., T.S., O.W. and S.A.L. cowrote the paper. S.C.K. and J.R. contributed equally to this paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Results (PDF 536 kb)
Supplementary Table 2
Selectivity Data of 3 (Kd = 7.6 nM for p38α) and Skepinone-L (5) (Kd = 1.5 nM for p38α) (XLS 83 kb)
Supplementary Table 3
Inhibitory activity data of Skepinone-L (IC50 = 2.8 nM) (XLS 46 kb)
Rights and permissions
About this article
Cite this article
Koeberle, S., Romir, J., Fischer, S. et al. Skepinone-L is a selective p38 mitogen-activated protein kinase inhibitor. Nat Chem Biol 8, 141–143 (2012). https://doi.org/10.1038/nchembio.761
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.761
This article is cited by
-
Decisive role of water and protein dynamics in residence time of p38α MAP kinase inhibitors
Nature Communications (2022)
-
Prediction of kinase inhibitors binding modes with machine learning and reduced descriptor sets
Scientific Reports (2021)
-
Exotoxins from Staphylococcus aureus activate 5-lipoxygenase and induce leukotriene biosynthesis
Cellular and Molecular Life Sciences (2020)
-
Communication between human macrophages and epithelial cancer cell lines dictates lipid mediator biosynthesis
Cellular and Molecular Life Sciences (2020)
-
Evaluation of the therapeutic potential of the selective p38 MAPK inhibitor Skepinone-L and the dual p38/JNK 3 inhibitor LN 950 in experimental K/BxN serum transfer arthritis
Inflammopharmacology (2019)