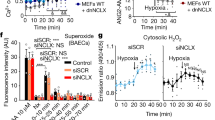
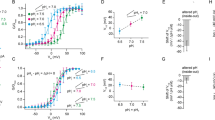
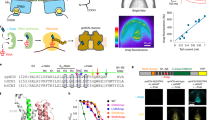
Abstract
Oxygen (O2) is a prerequisite for cellular respiration in aerobic organisms but also elicits toxicity. To understand how animals cope with the ambivalent physiological nature of O2, it is critical to elucidate the molecular mechanisms responsible for O2 sensing. Here our systematic evaluation of transient receptor potential (TRP) cation channels using reactive disulfides with different redox potentials reveals the capability of TRPA1 to sense O2. O2 sensing is based upon disparate processes: whereas prolyl hydroxylases (PHDs) exert O2-dependent inhibition on TRPA1 activity in normoxia, direct O2 action overrides the inhibition via the prominent sensitivity of TRPA1 to cysteine-mediated oxidation in hyperoxia. Unexpectedly, TRPA1 is activated through relief from the same PHD-mediated inhibition in hypoxia. In mice, disruption of the Trpa1 gene abolishes hyperoxia- and hypoxia-induced cationic currents in vagal and sensory neurons and thereby impedes enhancement of in vivo vagal discharges induced by hyperoxia and hypoxia. The results suggest a new O2-sensing mechanism mediated by TRPA1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Lane, N. Oxygen: The Molecule That Made the World (Oxford Univ. Press, Oxford, 2002).
Gonzalez, C., Almaraz, L., Obeso, A. & Rigual, R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol. Rev. 74, 829–898 (1994).
Neubauer, J.A. & Sunderram, J. Oxygen-sensing neurons in the central nervous system. J. Appl. Physiol. 96, 367–374 (2004).
Weir, E.K., López-Barneo, J., Buckler, K.J. & Archer, S.L. Acute oxygen-sensing mechanisms. N. Engl. J. Med. 353, 2042–2055 (2005).
Howe, A., Pack, R.J. & Wise, J.C. Arterial chemoreceptor-like activity in the abdominal vagus of the rat. J. Physiol. (Lond.) 320, 309–318 (1981).
De Sanctis, G.T., Green, F.H. & Remmers, J.E. Ventilatory responses to hypoxia and hypercapnia in awake rats pretreated with capsaicin. J. Appl. Physiol. 70, 1168–1174 (1991).
Gruss, M. et al. Moderate hypoxia influences excitability and blocks dendrotoxin sensitive K+ currents in rat primary sensory neurones. Mol. Pain 2, 12 (2006).
Longhurst, J.C. Tjen-A-Looi, S.C. & Fu, L.W. Cardiac sympathetic afferent activation provoked by myocardial ischemia and reperfusion. Mechanisms and reflexes. Ann. NY Acad. Sci. 940, 74–95 (2001).
Gray, J.M. et al. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature 430, 317–322 (2004).
Hetz, S.K. & Bradley, T.J. Insects breathe discontinuously to avoid oxygen toxicity. Nature 433, 516–519 (2005).
Clapham, D.E. TRP channels as cellular sensors. Nature 426, 517–524 (2003).
Voets, T., Talavera, K., Owsianik, G. & Nilius, B. Sensing with TRP channels. Nat. Chem. Biol. 1, 85–92 (2005).
Hara, Y. et al. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol. Cell 9, 163–173 (2002).
Yoshida, T. et al. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat. Chem. Biol. 2, 596–607 (2006).
Xu, S.Z. et al. TRPC channel activation by extracellular thioredoxin. Nature 451, 69–72 (2008).
Aarts, M. et al. A key role for TRPM7 channels in anoxic neuronal death. Cell 115, 863–877 (2003).
Bessac, B.F. et al. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J. Clin. Invest. 118, 1899–1910 (2008).
Hinman, A., Chuang, H.H., Bautista, D.M. & Julius, D. TRP channel activation by reversible covalent modification. Proc. Natl. Acad. Sci. USA 103, 19564–19568 (2006).
Macpherson, L.J. et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445, 541–545 (2007).
Takahashi, N. et al. Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels (Austin) 2, 287–298 (2008).
Story, G.M. et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112, 819–829 (2003).
Nagatomo, K. & Kubo, Y. Caffeine activates mouse TRPA1 channels but suppresses human TRPA1 channels. Proc. Natl. Acad. Sci. USA 105, 17373–17378 (2008).
Gracheva, E.O. et al. Molecular basis of infrared detection by snakes. Nature 464, 1006–1011 (2010).
Bessac, B.F. & Jordt, S.E. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda) 23, 360–370 (2008).
Caterina, M.J. et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 (1997).
Nagata, K., Duggan, A., Kumar, G. & García-Añoveros, J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J. Neurosci. 25, 4052–4061 (2005).
Nassenstein, C. et al. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J. Physiol. (Lond.) 586, 1595–1604 (2008).
Topol, I.A. et al. Experimental determination and calculations of redox potential descriptors of compounds directed against retroviral zinc fingers: Implications for rational drug design. Protein Sci. 10, 1434–1445 (2001).
Wallace, T.J., Schriesheim, A. & Bartok, W. The base-catalyzed oxidation of mercaptans. III. Role of the solvent and effect of mercaptan structure on the rate determining step. J. Org. Chem. 28, 1311–1314 (1963).
Petrus, M. et al. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol. Pain 3, 40 (2007).
Kim, D. & Cavanaugh, E.J. Requirement of a soluble intracellular factor for activation of transient receptor potential A1 by pungent chemicals: role of inorganic polyphosphates. J. Neurosci. 27, 6500–6509 (2007).
Schofield, C.J. & Ratcliffe, P.J. Oxygen sensing by HIF hydroxylases. Nat. Rev. Mol. Cell Biol. 5, 343–354 (2004).
Webb, J.D., Coleman, M.L. & Pugh, C.W. Hypoxia, hypoxia-inducible factors (HIF), HIF hydroxylases and oxygen sensing. Cell. Mol. Life Sci. 66, 3539–3554 (2009).
Bezzerides, V.J., Ramsey, I.S., Kotecha, S., Greka, A. & Clapham, D.E. Rapid vesicular translocation and insertion of TRP channels. Nat. Cell Biol. 6, 709–720 (2004).
Schmidt, M., Dubin, A.E., Petrus, M.J., Earley, T.J. & Patapoutian, A. Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron 64, 498–509 (2009).
Bandell, M. et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41, 849–857 (2004).
Kwan, K.Y. et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50, 277–289 (2006).
Aragonés, J. et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat. Genet. 40, 170–180 (2008).
Bishop, T. et al. Abnormal sympathoadrenal development and systemic hypotension in PHD3−/− mice. Mol. Cell. Biol. 28, 3386–3400 (2008).
Takeda, K. et al. Placental but not heart defects are associated with elevated hypoxia-inducible factor α levels in mice lacking prolyl hydroxylase domain protein 2. Mol. Cell. Biol. 26, 8336–8346 (2006).
Dinkova-Kostova, A.T. et al. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA 99, 11908–11913 (2002).
Hathout, Y. et al. Characterization of intermediates in the oxidation of zinc fingers in human immunodeficiency virus type 1 nucleocapsid protein P7. Drug Metab. Dispos. 24, 1395–1400 (1996).
Voss, A.A., Lango, J., Ernst-Russell, M., Morin, D. & Pessah, I.N. Identification of hyperreactive cysteines within ryanodine receptor type 1 by mass spectrometry. J. Biol. Chem. 279, 34514–34520 (2004).
Ghezzi, P. Regulation of protein function by glutathionylation. Free Radic. Res. 39, 573–580 (2005).
Hu, H., Bandell, M., Petrus, M.J., Zhu, M.X. & Patapoutian, A. Zinc activates damage-sensing TRPA1 ion channels. Nat. Chem. Biol. 5, 183–190 (2009).
Wang, Y.Y., Chang, R.B. & Liman, E.R. TRPA1 is a component of the nociceptive response to CO2 . J. Neurosci. 30, 12958–12963 (2010).
Semenza, G.L. & Wang, G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell. Biol. 12, 5447–5454 (1992).
Meller, S.T. & Gebhart, G.F. A critical review of the afferent pathways and the potential chemical mediators involved in cardiac pain. Neuroscience 48, 501–524 (1992).
Kubin, L., Alheid, G.F., Zuperku, E.J. & McCrimmon, D.R. Central pathways of pulmonary and lower airway vagal afferents. J. Appl. Physiol. 101, 618–627 (2006).
Mcelroy, M.B. The Atmospheric Environment: Effects of Human Activity (Princeton University Press, 2002).
Acknowledgements
We thank D. Julius, T. Yoshida and M. Wakamori for experimental advice, T. Miki and J. Ikenouchi for helpful discussions, and T. Morii and I. Hamachi for their support in DTNB-2Bio synthesis. We are also grateful to T. Niidome, H. Shirakawa and T. Nakagawa for their support in mouse experiments.
Author information
Authors and Affiliations
Contributions
N.T., S. Kiyonaka, Y. Mizuno and Y. Mori initiated and designed the project. N.T., S. Kiyonaka, T. Numata, D.K., Y. Mizuno, S.Y., S.N., T.O., S. Kaneko and T. Nokami performed experiments and analyzed data. T.K. supervised in vivo studies. S.S. and J.Y. supervised the electrochemical experiments. E.K. and P.C. established Phd1 knockout and Phd3 knockout mouse lines subjected to the experiment. N.T., T.K., S. Kiyonaka, T. Numata, D.K. and Y. Mori wrote the manuscript. Y. Mori directed the research. All authors discussed and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Results (PDF 12469 kb)
Rights and permissions
About this article
Cite this article
Takahashi, N., Kuwaki, T., Kiyonaka, S. et al. TRPA1 underlies a sensing mechanism for O2. Nat Chem Biol 7, 701–711 (2011). https://doi.org/10.1038/nchembio.640
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.640
This article is cited by
-
TRPA1 as a O2 sensor detects microenvironmental hypoxia in the mice anterior cingulate cortex
Scientific Reports (2023)
-
The human TRPA1 intrinsic cold and heat sensitivity involves separate channel structures beyond the N-ARD domain
Nature Communications (2022)
-
Linalool odor‐induced analgesia is triggered by TRPA1-independent pathway in mice
Behavioral and Brain Functions (2021)
-
Thiazoline-related innate fear stimuli orchestrate hypothermia and anti-hypoxia via sensory TRPA1 activation
Nature Communications (2021)
-
Oxygen-dependent regulation of ion channels: acute responses, post-translational modification, and response to chronic hypoxia
Pflügers Archiv - European Journal of Physiology (2021)