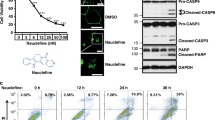
Abstract
Cephalostatin 1, OSW-1, ritterazine B and schweinfurthin A are natural products that potently, and in some cases selectively, inhibit the growth of cultured human cancer cell lines. The cellular targets of these small molecules have yet to be identified. We have discovered that these molecules target oxysterol binding protein (OSBP) and its closest paralog, OSBP-related protein 4L (ORP4L)—proteins not known to be involved in cancer cell survival. OSBP and the ORPs constitute an evolutionarily conserved protein superfamily, members of which have been implicated in signal transduction, lipid transport and lipid metabolism. The functions of OSBP and the ORPs, however, remain largely enigmatic. Based on our findings, we have named the aforementioned natural products ORPphilins. Here we used ORPphilins to reveal new cellular activities of OSBP. The ORPphilins are powerful probes of OSBP and ORP4L that will be useful in uncovering their cellular functions and their roles in human diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Pettit, G.R. et al. Antineoplastic agents. 147. Isolation and structure of the powerful cell growth inhibitor cephalostatin 1. J. Am. Chem. Soc. 110, 2006–2007 (1988).
Kubo, S. et al. Acylated cholestane glycosides from the bulbs of Ornithogalum saundersiae. Phytochemistry 31, 3969–3973 (1992).
Komiya, T. et al. Ritterazine B, a new cytotoxic natural compound, induces apoptosis in cancer cells. Cancer Chemother. Pharmacol. 51, 202–208 (2003).
Beutler, J.A., Shoemaker, R.H., Johnson, T. & Boyd, M.R. Cytotoxic geranyl stilbenes from Macaranga schweinfurthii. J. Nat. Prod. 61, 1509–1512 (1998).
Tasdemir, D. et al. Bioactive isomalabaricane triterpenes from the marine sponge Rhabdastrella globostellata. J. Nat. Prod. 65, 210–214 (2002).
Moser, B.R. Review of cytotoxic cephalostatins and ritterazines: isolation and synthesis. J. Nat. Prod. 71, 487–491 (2008).
Beutler, J.A. et al. The schweinfurthins. in Medicinal and Aromatic Plants. Agricultural, Commercial, Ecological, Legal, Pharmacological and Social Aspects Ch. 22 (eds. Bogers, R.J. et al.) (Springer, 2006).
Zhou, Y. et al. OSW-1: A natural compound with potent anticancer activity and a novel mechanism of action. J. Natl. Cancer Inst. 97, 1781–1785 (2005).
Rabow, A.A., Shoemaker, R.H., Sausville, E.A. & Covell, D.G. Mining the national cancer institute's tumor-screening database: identification of compounds with similar cellular activities. J. Med. Chem. 45, 818–840 (2002).
Mimaki, Y. et al. Cholestane glycosides with potent cytostatic activities on various tumor cells from Ornithogalum saundersiae bulbs. Bioorg. Med. Chem. Lett. 7, 633–636 (1997).
Boyd, M.R. The NCI Human Tumor Cell Line (60-Cell) Screen: Concept, Implementation, and Applications. in Anticancer Drug Development Guide: Preclinical Screening, Clinical Trials, and Approval Ch. 3 (eds. Teicher, B.A. et al.) (Humana Press, 2004).
Dirsch, V.M. et al. Cephalostatin 1 selectively triggers the release of Smac/DIABLO and subsequent apoptosis that is characterized by an increased density of the mitochondrial matrix. Cancer Res. 63, 8869–8876 (2003).
Rudy, A., López-Antón, N., Dirsch, V.M. & Vollmar, A.M. The cephalostatin way of apoptosis. J. Nat. Prod. 71, 482–486 (2008).
Turbyville, T.J. et al. Schweinfurthin A selectively inhibits proliferation and Rho signaling in glioma and neurofibromatosis type 1 tumor cells in a NF1-GRD–dependent manner. Mol. Cancer Ther. 9, 1234–1243 (2010).
Abbas, T. & Dutta, A. p21 in cancer: intricate networks and multiple activities. Nat. Rev. Cancer 9, 400–414 (2009).
Yan, D. & Olkkonen, V.M. Characteristics of oxysterol binding proteins. Int. Rev. Cytol. 265, 253–285 (2008).
Fairn, G.D. & McMaster, C.R. Emerging roles of the oxysterol-binding protein family in metabolism, transport, and signaling. Cell. Mol. Life Sci. 65, 228–236 (2008).
Yan, D. et al. Oxysterol binding protein induces upregulation of SREBP-1c and enhances hepatic lipogenesis. Arterioscler. Thromb. Vasc. Biol. 27, 1108–1114 (2007).
Yan, D. et al. Expression of human OSBP-related protein 1L in macrophages enhances atherosclerotic lesion development in LDL receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 27, 1618–1624 (2007).
Romeo, G.R. & Kazlauskas, A. Oxysterol and diabetes activate STAT3 and control endothelial expression of profilin-1 via OSBP1. J. Biol. Chem. 283, 9595–9605 (2008).
Wang, P.-Y., Weng, J. & Anderson, R.G.W. OSBP is a cholesterol-regulated scaffolding protein in control of ERK 1/2 activation. Science 307, 1472–1476 (2005).
Ngo, M. & Ridgway, N.D. Oxysterol binding protein–related protein 9 (ORP9) is a cholesterol transfer protein that regulates Golgi structure and function. Mol. Biol. Cell 20, 1388–1399 (2009).
Ngo, M.H., Colbourne, T.R. & Ridgway, N.D. Functional implications of sterol transport by the oxysterol-binding protein gene family. Biochem. J. 429, 13–24 (2010).
Schulz, T.A. et al. Lipid-regulated sterol transfer between closely apposed membranes by oxysterol-binding protein homologues. J. Cell Biol. 187, 889–903 (2009).
Taylor, F.R. & Kandutsch, A.A. Oxysterol binding protein. Chem. Phys. Lipids 38, 187–194 (1985).
Dawson, P.A., Ridgway, N.D., Slaughter, C.A., Brown, M.S. & Goldstein, J.L. cDNA cloning and expression of oxysterol-binding protein, an oligomer with a potential leucine zipper. J. Biol. Chem. 264, 16798–16803 (1989).
DeBerardinis, R.J., Sayed, N., Ditsworth, D. & Thompson, C.B. Brick by brick: metabolism and tumor cell growth. Curr. Opin. Genet. Dev. 18, 54–61 (2008).
Fortner, K.C., Kato, D., Tanaka, Y. & Shair, M.D. Enantioselective synthesis of (+)-cephalostatin 1. J. Am. Chem. Soc. 132, 275–280 (2010).
Wyles, J.P., McMaster, C.R. & Ridgway, N.D. Vesicle-associated membrane protein-associated protein-A (VAP-A) interacts with the oxysterol-binding protein to modify export from the endoplasmic reticulum. J. Biol. Chem. 277, 29908–29918 (2002).
Wyles, J.P. & Ridgway, N.D. VAMP-associated protein-A regulates partitioning of oxysterol-binding protein-related protein-9 between the endoplasmic reticulum and Golgi apparatus. Exp. Cell Res. 297, 533–547 (2004).
Wyles, J.P., Perry, R.J. & Ridgway, N.D. Characterization of the sterol-binding domain of oxysterol-binding protein (OSBP)-related protein 4 reveals a novel role in vimentin organization. Exp. Cell Res. 313, 1426–1437 (2007).
Infante, R.E. et al. Purified NPC1 protein. I. Binding of cholesterol and oxysterols to a 1278-amino acid membrane protein. J. Biol. Chem. 283, 1052–1063 (2008).
Radhakrishnan, A., Ikeda, Y., Kwon, H.J., Brown, M.S. & Goldstein, J.L. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: oxysterols block transport by binding to Insig. Proc. Natl. Acad. Sci. USA 104, 6511–6518 (2007).
Infante, R.E. et al. NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc. Natl. Acad. Sci. USA 105, 15287–15292 (2008).
Metherall, J.E., Goldstein, J.L., Luskey, K.L. & Brown, M.S. Loss of transcriptional repression of three sterol-regulated genes in mutant hamster cells. J. Biol. Chem. 264, 15634–15641 (1989).
Wang, P.-Y., Weng, J., Lee, S. & Anderson, R.G.W. The N terminus controls sterol binding while the C terminus regulates the scaffolding function of OSBP. J. Biol. Chem. 283, 8034–8045 (2008).
Ridgway, N.D., Dawson, P.A., Ho, Y.K., Brown, M.S. & Goldstein, J.L. Translocation of oxysterol binding protein to Golgi apparatus triggered by ligand binding. J. Cell Biol. 116, 307–319 (1992).
Nishimura, T. et al. Inhibition of cholesterol biosynthesis by 25-hydroxycholesterol is independent of OSBP. Genes Cells 10, 793–801 (2005).
El Khissiin, A. & Leclercq, G. Implication of proteasome in estrogen receptor degradation. FEBS Lett. 448, 160–166 (1999).
Perry, R.J. & Ridgway, N.D. Oxysterol-binding protein and vesicle-associated membrane protein–associated protein are required for sterol-dependent activation of the ceramide transport protein. Mol. Biol. Cell 17, 2604–2616 (2006).
Kawano, M., Kumagai, K., Nishijima, M. & Hanada, K. Efficient trafficking of ceramide from the endoplasmic reticulum to the Golgi apparatus requires a VAMP-associated protein-interacting FFAT motif of CERT. J. Biol. Chem. 281, 30279–30288 (2006).
Peretti, D., Dahan, N., Shimoni, E., Hirschberg, K. & Lev, S. Coordinated lipid transfer between the endoplasmic reticulum and the Golgi complex requires the VAP proteins and is essential for Golgi-mediated transport. Mol. Biol. Cell 19, 3871–3884 (2008).
Ridgway, N.D. 25-Hydroxycholesterol stimulates sphingomyelin synthesis in Chinese hamster ovary cells. J. Lipid Res. 36, 1345–1358 (1995).
Collingwood, T.N. et al. A natural transactivation mutation in the thyroid hormone β receptor: Impaired interaction with putative transcriptional mediators. Proc. Natl. Acad. Sci. USA 94, 248–253 (1997).
Beh, C.T., Cool, L., Phillips, J. & Rine, J. Overlapping functions of the yeast oxysterol-binding protein homologues. Genetics 157, 1117–1140 (2001).
Suchanek, M. et al. The mammalian oxysterol-binding protein-related proteins (ORPs) bind 25-hydroxycholesterol in an evolutionarily conserved pocket. Biochem. J. 405, 473–480 (2007).
Olkkonen, V.M. & Hynynen, R. Interactions of oxysterols with membranes and proteins. Mol. Aspects Med. 30, 123–133 (2009).
Zerbinatti, C.V. et al. Oxysterol-binding protein-1 (OSBP1) modulates processing and trafficking of the amyloid precursor protein. Mol. Neurodegener. 3, 5 (2008).
Fournier, M.V. et al. Identification of a gene encoding a human oxysterol-binding protein-homologue: a potential general molecular marker for blood dissemination of solid tumors. Cancer Res. 59, 3748–3753 (1999), erratum 61, 792 (2001).
Acknowledgements
We thank the NCI for the generous gifts of schweinfurthin A. (J.A. Beutler) and ritterazine B. We gratefully acknowledge the aforementioned reagents provided by G. Romeo (Joslin Diabetes Center), M. Brown and J. Goldstein (University of Texas Southwestern Medical Center), H. Arai (University of Tokyo), B. Vogelstein (Johns Hopkins University) and D. Ory (Washington University, St. Louis). The beta-tubulin monoclonal antibody (E7) developed by M. Klymkowsky was obtained from the Developmental Studies Hybridoma Bank (National Institute of Child Health and Human Development, US National Institutes of Health (NIH) and University of Iowa, Department of Biology). R. King is acknowledged for helpful comments and discussions. T.B.P. would like to thank the Lundbeck foundation for a post-doctoral fellowship and the Danish Council for Independent Research–Natural Sciences for additional financial support. A.W.G.B gratefully thanks the Susan G. Komen for the Cure Foundation for providing a postdoctoral research fellowship. Financial support from the Novartis Institutes of Biomedical Research, the US NIH (grant no. R01GM090068), the Harvard Catalyst and The Harvard Clinical and Translational Science Center (NIH award no. UL1RR025758) is acknowledged. This work was conducted with support and financial contributions from Harvard University and its affiliated academic health care centers.
Author information
Authors and Affiliations
Contributions
M.D.S., A.W.G.B., T.B.P., K.W. and D.R.A. conceived and designed the study. A.W.G.B., T.B.P., K.W. and D.R.A. implemented experiments. K.C.F. prepared cephalostatin 1. The OSBP and ORP4L molecular biology was done by K.W. and C.K. K.S. prepared OSW-1. S.O. performed early studies on p21 selectivity. Y.M. and M.K. supplied OSW-1 isolated from nature. A.W.G.B., D.R.A., M.S., J.P.M., E.C.P., D.J.S., I.C.-T. and J.A.T. performed the OSW-1 affinity chromatography experiments and subsequent quantitative mass spectrometry analysis. M.D.S., A.W.G.B., T.B.P. and K.W. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Results (PDF 1238 kb)
Rights and permissions
About this article
Cite this article
Burgett, A., Poulsen, T., Wangkanont, K. et al. Natural products reveal cancer cell dependence on oxysterol-binding proteins. Nat Chem Biol 7, 639–647 (2011). https://doi.org/10.1038/nchembio.625
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.625
This article is cited by
-
Single-molecule localization microscopy reveals STING clustering at the trans-Golgi network through palmitoylation-dependent accumulation of cholesterol
Nature Communications (2024)
-
Targeting lipid–protein interaction to treat Syk-mediated acute myeloid leukemia
Nature Chemical Biology (2023)
-
An acquired phosphatidylinositol 4-phosphate transport initiates T-cell deterioration and leukemogenesis
Nature Communications (2022)
-
RNAseq reveals extensive metabolic disruptions in the sensitive SF-295 cell line treated with schweinfurthins
Scientific Reports (2022)
-
OSW-1 induces apoptosis and cyto-protective autophagy, and synergizes with chemotherapy on triple negative breast cancer metastasis
Cellular Oncology (2022)