Abstract
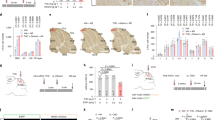
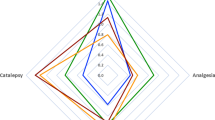
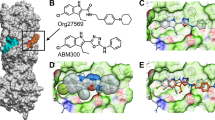
Cannabinoids enhance the function of glycine receptors (GlyRs). However, little is known about the mechanisms and behavioral implication of cannabinoid-GlyR interaction. Using mutagenesis and NMR analysis, we have identified a serine at 296 in the GlyR protein critical for the potentiation of IGly by Δ9-tetrahydrocannabinol (THC), a major psychoactive component of marijuana. The polarity of the amino acid residue at 296 and the hydroxyl groups of THC are critical for THC potentiation. Removal of the hydroxyl groups of THC results in a compound that does not affect IGly when applied alone but selectively antagonizes cannabinoid-induced potentiating effect on IGly and analgesic effect in a tail-flick test in mice. The cannabinoid-induced analgesia is absent in mice lacking α3GlyRs but not in those lacking CB1 and CB2 receptors. These findings reveal a new mechanism underlying cannabinoid potentiation of GlyRs, which could contribute to some of the cannabis-induced analgesic and therapeutic effects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Pacher, P., Batkai, S. & Kunos, G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 58, 389–462 (2006).
Zimmer, A., Zimmer, A.M., Hohmann, A.G., Herkenham, M. & Bonner, T.I. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc. Natl. Acad. Sci. USA 96, 5780–5785 (1999).
Oz, M. Receptor-independent actions of cannabinoids on cell membranes: focus on endocannabinoids. Pharmacol. Ther. 111, 114–144 (2006).
Zhang, L. & Xiong, W. Modulation of the Cys-loop ligand-gated ion channels by fatty acid and cannabinoids. Vitam. Horm. 81, 315–335 (2009).
Welch, S.P., Huffman, J.W. & Lowe, J. Differential blockade of the antinociceptive effects of centrally administered cannabinoids by SR141716A. J. Pharmacol. Exp. Ther. 286, 1301–1308 (1998).
Pernía-Andrade, A.J. et al. Spinal endocannabinoids and CB1 receptors mediate C-fiber-induced heterosynaptic pain sensitization. Science 325, 760–764 (2009).
Zhang, G., Chen, W., Lao, L. & Marvizon, J.C. Cannabinoid CB1 receptor facilitation of substance P release in the rat spinal cord, measured as neurokinin 1 receptor internalization. Eur. J. Neurosci. 31, 225–237 (2010).
Klein, T.W. & Newton, C.A. Therapeutic potential of cannabinoid-based drugs. Adv. Exp. Med. Biol. 601, 395–413 (2007).
Izzo, A.A., Borrelli, F., Capasso, R., Di Marzo, V. & Mechoulam, R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 30, 515–527 (2009).
Hejazi, N. et al. Delta9-tetrahydrocannabinol and endogenous cannabinoid anandamide directly potentiate the function of glycine receptors. Mol. Pharmacol. 69, 991–997 (2006).
Yang, Z. et al. Subunit-specific modulation of glycine receptors by cannabinoids and N-arachidonyl-glycine. Biochem. Pharmacol. 76, 1014–1023 (2008).
Demir, R. et al. Modulation of glycine receptor function by the synthetic cannabinoid HU210. Pharmacology 83, 270–274 (2009).
Lynch, J.W. Native glycine receptor subtypes and their physiological roles. Neuropharmacology 56, 303–309 (2009).
Shiang, R. et al. Mutational analysis of familial and sporadic hyperekplexia. Ann. Neurol. 38, 85–91 (1995).
Shiang, R. et al. Mutations in the alpha 1 subunit of the inhibitory glycine receptor cause the dominant neurologic disorder, hyperekplexia. Nat. Genet. 5, 351–358 (1993).
Harvey, R.J. et al. GlyR alpha3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science 304, 884–887 (2004).
Griffon, N. et al. Molecular determinants of glycine receptor subunit assembly. EMBO J. 18, 4711–4721 (1999).
Jordt, S.E. et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427, 260–265 (2004).
Kimura, T., Cheng, K., Rice, K.C. & Gawrisch, K. Location, structure, and dynamics of the synthetic cannabinoid ligand CP-55,940 in lipid bilayers. Biophys. J. 96, 4916–4924 (2009).
Grossman, M.L., Basbaum, A.I. & Fields, H.L. Afferent and efferent connections of the rat tail flick reflex (a model used to analyze pain control mechanisms). J. Comp. Neurol. 206, 9–16 (1982).
Lobo, I.A. & Harris, R.A. Sites of alcohol and volatile anesthetic action on glycine receptors. Int. Rev. Neurobiol. 65, 53–87 (2005).
Harris, R.A., Trudell, J.R. & Mihic, S.J. Ethanol's molecular targets. Sci. Signal. 1, re7 (2008).
Smith, P.B. & Martin, B.R. Spinal mechanisms of delta 9-tetrahydrocannabinol-induced analgesia. Brain Res. 578, 8–12 (1992).
Compton, D.R., Aceto, M.D., Lowe, J. & Martin, B.R. In vivo characterization of a specific cannabinoid receptor antagonist (SR141716A): inhibition of delta 9-tetrahydrocannabinol-induced responses and apparent agonist activity. J. Pharmacol. Exp. Ther. 277, 586–594 (1996).
Varvel, S.A. et al. Delta9-tetrahydrocannbinol accounts for the antinociceptive, hypothermic, and cataleptic effects of marijuana in mice. J. Pharmacol. Exp. Ther. 314, 329–337 (2005).
Marris, E. More pain studies needed. Nature 458, 394–395 (2009).
Ledent, C. et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science 283, 401–404 (1999).
Zeilhofer, H.U. The glycinergic control of spinal pain processing. Cell. Mol. Life Sci. 62, 2027–2035 (2005).
Zeilhofer, H.U. Prostanoids in nociception and pain. Biochem. Pharmacol. 73, 165–174 (2007).
Findlay, G.S. et al. Glycine receptor knock-in mice and hyperekplexia-like phenotypes: comparisons with the null mutant. J. Neurosci. 23, 8051–8059 (2003).
Huestis, M.A. Human cannabinoid pharmacokinetics. Chem. Biodivers. 4, 1770–1804 (2007).
Spivak, C.E., Lupica, C.R. & Oz, M. The endocannabinoid anandamide inhibits the function of alpha4beta2 nicotinic acetylcholine receptors. Mol. Pharmacol. 72, 1024–1032 (2007).
Xiong, W., Hosoi, M., Koo, B.N. & Zhang, L. Anandamide inhibition of 5–HT3A receptors varies with receptor density and desensitization. Mol. Pharmacol. 73, 314–322 (2008).
Ahrens, J. et al. The nonpsychotropic cannabinoid cannabidiol modulates and directly activates alpha-1 and alpha-1-Beta glycine receptor function. Pharmacology 83, 217–222 (2009).
Laube, B., Maksay, G., Schemm, R. & Betz, H. Modulation of glycine receptor function: a novel approach for therapeutic intervention at inhibitory synapses? Trends Pharmacol. Sci. 23, 519–527 (2002).
Delaney, A.J., Esmaeili, A., Sedlak, P.L., Lynch, J.W. & Sah, P. Differential expression of glycine receptor subunits in the rat basolateral and central amygdala. Neurosci. Lett. 469, 237–242 (2010).
Buckley, N.E. et al. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur. J. Pharmacol. 396, 141–149 (2000).
Young-Pearse, T.L., Ivic, L., Kriegstein, A.R. & Cepko, C.L. Characterization of mice with targeted deletion of glycine receptor alpha 2. Mol. Cell. Biol. 26, 5728–5734 (2006).
Hu, X.Q., Zhang, L., Stewart, R.R. & Weight, F.F. Arginine 222 in the pre-transmembrane domain 1 of 5–HT3A receptors links agonist binding to channel gating. J. Biol. Chem. 278, 46583–46589 (2003).
Arnold, K., Bordoli, L., Kopp, J. & Schwede, T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22, 195–201 (2006).
Hilf, R.J. & Dutzler, R. Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature 457, 115–118 (2009).
Canlas, C.G., Cui, T., Li, L., Xu, Y. & Tang, P. Anesthetic modulation of protein dynamics: insight from an NMR study. J. Phys. Chem. B 112, 14312–14318 (2008).
Ma, D., Liu, Z., Li, L., Tang, P. & Xu, Y. Structure and dynamics of the second and third transmembrane domains of human glycine receptor. Biochemistry 44, 8790–8800 (2005).
Tang, P., Mandal, P.K. & Xu, Y. NMR structures of the second transmembrane domain of the human glycine receptor alpha(1) subunit: model of pore architecture and channel gating. Biophys. J. 83, 252–262 (2002).
Acknowledgements
We thank C.L. Cepko for providing α2−/− Glra mice. We especially thank D.M. Lovinger for critical comments on the manuscript. This work was supported by funds from the intramural program of the US National Institute on Alcohol Abuse and Alcoholism. We acknowledge the grant support (R37GM049202 to Y.X.) from the US National Institute of General Medical Sciences of the US National Institutes of Health.
Author information
Authors and Affiliations
Contributions
W.X. and L.Z. designed and conducted electrophysiology, biochemical and biophysical experiments and animal behavioral tests. K.-J.C. and K.C.R. synthesized cannabinoid chemicals. T-X.C. and Y.X. conducted protein expression, purification and NMR experiments of the full-length transmembrane domains of the α1 GlyR and carried out NMR analysis. G.G. conducted animal behavioral tests. L.Z. supervised the project and wrote the manuscript. Y.X. critically revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Figures 1–6 (PDF 868 kb)
Rights and permissions
About this article
Cite this article
Xiong, W., Cheng, K., Cui, T. et al. Cannabinoid potentiation of glycine receptors contributes to cannabis-induced analgesia. Nat Chem Biol 7, 296–303 (2011). https://doi.org/10.1038/nchembio.552
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.552
This article is cited by
-
Schisandrin B, a dual positive allosteric modulator of GABAA and glycine receptors, alleviates seizures in multiple mouse models
Acta Pharmacologica Sinica (2024)
-
Excitatory and inhibitory neuronal signaling in inflammatory and diabetic neuropathic pain
Molecular Medicine (2023)
-
Combined alcohol and cannabinoid exposure leads to synergistic toxicity by affecting cerebellar Purkinje cells
Nature Metabolism (2022)
-
Structural basis for cannabinoid-induced potentiation of alpha1-glycine receptors in lipid nanodiscs
Nature Communications (2022)
-
Cannabidiol as a modulator of α7 nicotinic receptors
Cellular and Molecular Life Sciences (2022)