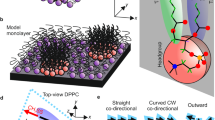
Abstract
We document a new dimension of surface recognition in which communication is controlled through the collective behavior of lipids. Membrane cholesterol induces a tilt in glycolipid receptor headgroup, resulting in loss of access for ligand binding. This property appears to organize erythrocyte blood group presentation and glycolipid receptor function during the activation of sperm fertility, suggesting that lipid 'allostery' is a means to regulate membrane recognition processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hakomori, S.I. Biochim. Biophys. Acta 1780, 325–346 (2008).
Lingwood, C.A. Glycoconj. J. 13, 495–503 (1996).
Evans, S.V. & MacKenzie, C.R. J. Mol. Recognit. 12, 155–168 (1999).
Shi, J.J., Yang, T.L. & Cremer, P.S. J. Am. Chem. Soc. 129, 5954–5961 (2007).
Hatzakis, N.S. et al. Nat. Chem. Biol. 5, 835–841 (2009).
Merritt, E.A. et al. Protein Sci. 6, 1516–1528 (1997).
Khan, F., Proulx, F. & Lingwood, C.A. Kidney Int. 75, 1209–1216 (2009).
Bitzan, M. Infect. Immun. 62, 3337–3347 (1994).
Huflejt, M.E. et al. Mol. Immunol. 46, 3037–3049 (2009).
Bedford, J.M. Int. J. Dev. Biol. 52, 415–426 (2008).
Cross, N.L. Biol. Reprod. 59, 7–11 (1998).
Lingwood, D. & Simons, K. Science 327, 46–50 (2010).
Jones, R. et al. Dev. Biol. 339, 398–406 (2010).
White, D. et al. Biol. Reprod. 63, 147–155 (2000).
Weerachatyanukul, W. et al. Mol. Reprod. Dev. 60, 569–578 (2001).
Visconti, P.E. et al. J. Biol. Chem. 274, 3235–3242 (1999).
Kawano, N., Yoshida, K., Iwamoto, T. & Yoshida, M. Biol. Reprod. 79, 1153–1159 (2008).
Selvaraj, V. et al. J. Cell. Physiol. 206, 636–646 (2006).
Selvaraj, V. et al. J. Androl. 28, 588–599 (2007).
Strott, C.A. & Higashi, Y. J. Lipid Res. 44, 1268–1278 (2003).
Mahfoud, R. et al. J. Biol. Chem. 285, 36049–36059 (2010).
Yahi, N., Aulas, A. & Fantini, J. PLoS ONE 5, e9079 (2010).
Acknowledgements
The authors thank I. Nuesslein, M. Gerl, I. Levental, H. Kaiser (Max Planck Institute for Molecular Cell Biology and Genetics), K. Hölig (Technische Univesität), P. Paroutis, M. Woodside (Hospital for Sick Children), U. Devi, W. Jones and M. Swann (Farfield Sensors) for their assistance in this project. This work was supported by a MPI-CBG stipend (D.L.) and funding to K.S. (EU FP6 Lipid PRISM Grant LSHB-CT2007-037740, DFG Schwerpunktprogramm 1175 Grant SI459/2-1, DFG Transregio 83 Grant: TRR83 TP02, BMBF ForMaT Grant: 03FO1212, ESF “LIPIDPROD” Grant: SI459/3-1); I.V. (Academy of Finland); and funding to C.A.L. (Canadian Institutes of Health Research Grant MT 13747, Ontario HIV Treatment Network and Canfar support).
Author information
Authors and Affiliations
Contributions
Membrane and sperm recognition experiments were performed by D.L.; histology was by B.B.; molecular dynamics simulations were by T.R. and I.V.; DPI was performed by D.L., M.G. and U.C.; D.L., C.A.L. and K.S. formulated the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Figures 1–10 (PDF 1001 kb)
Rights and permissions
About this article
Cite this article
Lingwood, D., Binnington, B., Róg, T. et al. Cholesterol modulates glycolipid conformation and receptor activity. Nat Chem Biol 7, 260–262 (2011). https://doi.org/10.1038/nchembio.551
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.551
This article is cited by
-
The bacterial lectin LecA from P. aeruginosa alters membrane organization by dispersing ordered domains
Communications Physics (2023)
-
Recently developed glycosphingolipid probes and their dynamic behavior in cell plasma membranes as revealed by single-molecule imaging
Glycoconjugate Journal (2023)
-
Cell density-dependent membrane distribution of ganglioside GM3 in melanoma cells
Cellular and Molecular Life Sciences (2023)
-
Differential recognition of lipid domains by two Gb3-binding lectins
Scientific Reports (2020)
-
The effects of dyslipidaemia and cholesterol modulation on erythrocyte susceptibility to malaria parasite infection
Malaria Journal (2019)