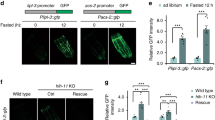
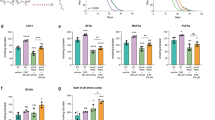
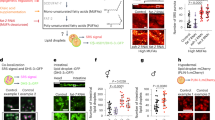
Abstract
The regulation of energy homeostasis integrates diverse biological processes ranging from behavior to metabolism and is linked fundamentally to numerous disease states. To identify new molecules that can bypass homeostatic compensatory mechanisms of energy balance in intact animals, we screened for small-molecule modulators of Caenorhabditis elegans fat content. We report on several molecules that modulate fat storage without obvious deleterious effects on feeding, growth and reproduction. A subset of these compounds also altered fat storage in mammalian and insect cell culture. We found that one of the newly identified compounds exerts its effects in C. elegans through a pathway that requires previously undescribed functions of an AMP-activated kinase catalytic subunit and a transcription factor previously unassociated with fat regulation. Thus, our strategy identifies small molecules that are effective within the context of intact animals and reveals relationships between new pathways that operate across phyla to influence energy homeostasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Spiegelman, B.M. & Flier, J.S. Obesity and the regulation of energy balance. Cell 104, 531–543 (2001).
Kopelman, P.G. Obesity as a medical problem. Nature 404, 635–643 (2000).
Knight, Z.A. & Shokat, K.M. Chemical genetics: where genetics and pharmacology meet. Cell 128, 425–430 (2007).
Stelling, J. et al. Robustness of cellular functions. Cell 118, 675–685 (2004).
Wheeler, G.N. & Brandli, A.W. Simple vertebrate models for chemical genetics and drug discovery screens: Lessons from zebrafish and Xenopus. Dev. Dyn. 238, 1287–1308 (2009).
Moy, T.I. et al. High-throughput screen for novel antimicrobials using a whole animal infection model. ACS Chem. Biol. 4, 527–533 (2009).
Petrascheck, M., Ye, X. & Buck, L.B. An antidepressant that extends lifespan in adult Caenorhabditis elegans. Nature 450, 553–556 (2007).
Kwok, T.C. et al. A small-molecule screen in C. elegans yields a new calcium channel antagonist. Nature 441, 91–95 (2006).
Jones, A.K., Buckingham, S.D. & Sattelle, D.B. Chemistry-to-gene screens in Caenorhabditis elegans. Nat. Rev. Drug Discov. 4, 321–330 (2005).
Schafer, W.R., Sanchez, B.M. & Kenyon, C.J. Genes affecting sensitivity to serotonin in Caenorhabditis elegans. Genetics 143, 1219–1230 (1996).
Weinshenker, D., Garriga, G. & Thomas, J.H. Genetic and pharmacological analysis of neurotransmitters controlling egg laying in C. elegans. J. Neurosci. 15, 6975–6985 (1995).
Watts, J.L. Fat synthesis and adiposity regulation in Caenorhabditis elegans. Trends Endocrinol. Metab. 20, 58–65 (2009).
Perez, C.L. & Van Gilst, M.R. A 13C isotope labeling strategy reveals the influence of insulin signaling on lipogenesis in C. elegans. Cell Metab. 8, 266–274 (2008).
Jones, K.T. & Ashrafi, K. Caenorhabditis elegans as an emerging model for studying the basic biology of obesity. Dis. Model. Mech. 2, 224–229 (2009).
McKay, R.M. et al. C. elegans: a model for exploring the genetics of fat storage. Dev. Cell 4, 131–142 (2003).
Jia, K., Chen, D. & Riddle, D.L. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development 131, 3897–3906 (2004).
Kimura, K.D. et al. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277, 942–946 (1997).
Srinivasan, S. et al. Serotonin regulates C. elegans fat and feeding through independent molecular mechanisms. Cell Metab. 7, 533–544 (2008).
de Bono, M. & Bargmann, C.I. Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell 94, 679–689 (1998).
Suo, S., Culotti, J.G. & Van Tol, H.H. Dopamine counteracts octopamine signalling in a neural circuit mediating food response in C. elegans. EMBO J. 28, 2437–2448 (2009).
Ashrafi, K. et al. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature 421, 268–272 (2003).
Greenspan, P., Mayer, E.P. & Fowler, S.D. Nile red: a selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 100, 965–973 (1985).
Van Gilst, M.R. et al. Nuclear hormone receptor NHR-49 controls fat consumption and fatty acid composition in C. elegans. PLoS Biol. 3, e53 (2005).
Jones, K.S. et al. A high throughput live transparent animal bioassay to identify non-toxic small molecules or genes that regulate vertebrate fat metabolism for obesity drug development. Nutr. Metab. (Lond) 5, 23 (2008).
Sullivan, J.E. et al. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett. 353, 33–36 (1994).
Watts, J.L. & Browse, J. A palmitoyl-CoA-specific delta9 fatty acid desaturase from Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 272, 263–269 (2000).
Van Gilst, M.R., Hadjivassiliou, H. & Yamamoto, K.R. A Caenorhabditis elegans nutrient response system partially dependent on nuclear receptor NHR-49. Proc. Natl. Acad. Sci. USA 102, 13496–13501 (2005).
Yang, F. et al. An ARC/Mediator subunit required for SREBP control of cholesterol and lipid homeostasis. Nature 442, 700–704 (2006).
MacDougald, O.A. & Lane, M.D. Transcriptional regulation of gene expression during adipocyte differentiation. Annu. Rev. Biochem. 64, 345–373 (1995).
Guo, Y. et al. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature 453, 657–661 (2008).
Horton, J.D. et al. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc. Natl. Acad. Sci. USA 100, 12027–12032 (2003).
Kniazeva, M. et al. Monomethyl branched-chain fatty acids play an essential role in Caenorhabditis elegans development. PLoS Biol. 2, E257 (2004).
Shiri-Sverdlov, R. et al. Identification of TUB as a novel candidate gene influencing body weight in humans. Diabetes 55, 385–389 (2006).
Goldstone, A.P. & Beales, P.L. Genetic obesity syndromes. Front. Horm. Res. 36, 37–60 (2008).
Ogg, S. et al. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389, 994–999 (1997).
Steinberg, G.R. & Kemp, B.E. AMPK in health and disease. Physiol. Rev. 89, 1025–1078 (2009).
Apfeld, J. et al. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 18, 3004–3009 (2004).
Narbonne, P. & Roy, R. Caenorhabditis elegans dauers need LKB1/AMPK to ration lipid reserves and ensure long-term survival. Nature 457, 210–214 (2009).
Dobrzyn, P. et al. Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver. Proc. Natl. Acad. Sci. USA 101, 6409–6414 (2004).
Corton, J.M. et al. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur. J. Biochem. 229, 558–565 (1995).
Owen, M.R., Doran, E. & Halestrap, A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 348, 607–614 (2000).
Cool, B. et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 3, 403–416 (2006).
Wang, X., Jia, H. & Chamberlin, H.M. The bZip proteins CES-2 and ATF-2 alter the timing of transcription for a cell-specific target gene in C. elegans. Dev. Biol. 289, 456–465 (2006).
Zhou, G. et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108, 1167–1174 (2001).
Davies, S.P., Sim, A.T. & Hardie, D.G. Location and function of three sites phosphorylated on rat acetyl-CoA carboxylase by the AMP-activated protein kinase. Eur. J. Biochem. 187, 183–190 (1990).
O'Rourke, E.J. et al. C. elegans major fats are stored in vesicles distinct from lysosome-related organelles. Cell Metab. 10, 430–435 (2009).
Brooks, K.K., Liang, B. & Watts, J.L. The influence of bacterial diet on fat storage in C. elegans. PLoS ONE 4, e7545 (2009).
Mörck, C. et al. Statins inhibit protein lipidation and induce the unfolded protein response in the non-sterol producing nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 106, 18285–18290 (2009).
Kodiha, M. et al. Localization of AMP kinase is regulated by stress, cell density, and signaling through the MEK→ERK1/2 pathway. Am. J. Physiol. Cell Physiol. 293, C1427–C1436 (2007).
MacRae, C.A. & Peterson, R.T. Zebrafish-based small molecule discovery. Chem. Biol. 10, 901–908 (2003).
Acknowledgements
We are grateful to D. Lum for his help and advice with the initial C. elegans experiments. We are thankful to B. Mullaney and K. Cunningham for helpful discussions and comments on the manuscript. We thank K. Thorn and the Nikon Imaging Center for use of the Nikon 6D automated epifluorescence microscope and help with imaging as well as the Small Molecule Discovery Center at the University of California, San Francisco, for providing the small-molecule library. This work was supported by grants from the US National Cancer Institute and US National Institute of Environmental Health Sciences (ES012801, ES019458 and CA056721) to Z.W., the US National Institute of Diabetes and Digestive and Kidney Diseases (DK070149) to K.A., the US National Institute of General Medicine (GM081863) to R.J.B. and a Byers Award to K.A. and R.J.B.
Author information
Authors and Affiliations
Contributions
G.A.L., K.A. and Z.W. conceived the study design. G.A.L., J.L. and N.M. performed the experiments. G.A.L., K.A., R.J.B. and Z.W. analyzed the data. G.A.L., K.A. and Z.W. wrote the paper. All the authors read, revised and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Figures 1–7 (PDF 2675 kb)
Rights and permissions
About this article
Cite this article
Lemieux, G., Liu, J., Mayer, N. et al. A whole-organism screen identifies new regulators of fat storage. Nat Chem Biol 7, 206–213 (2011). https://doi.org/10.1038/nchembio.534
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.534
This article is cited by
-
Picroscope: low-cost system for simultaneous longitudinal biological imaging
Communications Biology (2021)
-
Investigation of anthelmintic activity of the acetone extract and constituents of Typha capensis against animal parasitic Haemonchus contortus and free-living Caenorhabditis elegans
Parasitology Research (2021)
-
A robust and miniaturized screening platform to study natural products affecting metabolism and survival in Caenorhabditis elegans
Scientific Reports (2020)
-
Novel coumarins active against Trypanosoma cruzi and toxicity assessment using the animal model Caenorhabditis elegans
BMC Pharmacology and Toxicology (2019)
-
Sequestration of ubiquitous dietary derived pigments enables mitochondrial light sensing
Scientific Reports (2016)