Abstract
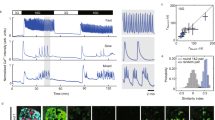
Many protein kinases are key nodal signaling molecules that regulate a wide range of cellular functions. These functions may require complex spatiotemporal regulation of kinase activities. Here, we show that protein kinase A (PKA), Ca2+ and cyclic AMP (cAMP) oscillate in sync in insulin-secreting MIN6 beta cells, forming a highly integrated oscillatory circuit. We found that PKA activity was essential for this oscillatory circuit and was capable of not only initiating the signaling oscillations but also modulating their frequency, thereby diversifying the spatiotemporal control of downstream signaling. Our findings suggest that exquisite temporal control of kinase activity, mediated via signaling circuits resulting from cross-regulation of signaling pathways, can encode diverse inputs into temporal parameters such as oscillation frequency, which in turn contribute to proper regulation of complex cellular functions in a context-dependent manner.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Manning, G., Whyte, D.B., Martinez, R., Hunter, T. & Sudarsanam, S. The protein kinase complement of the human genome. Science 298, 1912–1934 (2002).
Ubersax, J.A. & Ferrell, J.E. Jr. Mechanisms of specificity in protein phosphorylation. Nat. Rev. Mol. Cell Biol. 8, 530–541 (2007).
Carnegie, G.K., Means, C.K. & Scott, J.D. A-kinase anchoring proteins: from protein complexes to physiology and disease. IUBMB Life 61, 394–406 (2009).
Brown, M.D. & Sacks, D.B. Protein scaffolds in MAP kinase signalling. Cell. Signal. 21, 462–469 (2009).
Marshall, C.J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80, 179–185 (1995).
Taylor, S.S. et al. Signaling through cAMP and cAMP-dependent protein kinase: diverse strategies for drug design. Biochim. Biophys. Acta 1784, 16–26 (2008).
Seino, S. & Shibasaki, T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol. Rev. 85, 1303–1342 (2005).
Bertram, R., Sherman, A. & Satin, L.S. Metabolic and electrical oscillations: partners in controlling pulsatile insulin secretion. Am. J. Physiol. Endocrinol. Metab. 293, E890–E900 (2007).
Gromada, J., Brock, B., Schmitz, O. & Rorsman, P. Glucagon-like peptide-1: regulation of insulin secretion and therapeutic potential. Basic Clin. Pharmacol. Toxicol. 95, 252–262 (2004).
Delmeire, D. et al. Type VIII adenylyl cyclase in rat beta cells: coincidence signal detector/generator for glucose and GLP-1. Diabetologia 46, 1383–1393 (2003).
Henquin, J.C. & Meissner, H.P. The ionic, electrical, and secretory effects of endogenous cyclic adenosine monophosphate in mouse pancreatic B cells: studies with forskolin. Endocrinology 115, 1125–1134 (1984).
Ammälä, C., Ashcroft, F.M. & Rorsman, P. Calcium-independent potentiation of insulin release by cyclic AMP in single beta-cells. Nature 363, 356–358 (1993).
Hatakeyama, H., Kishimoto, T., Nemoto, T., Kasai, H. & Takahashi, N. Rapid glucose sensing by protein kinase A for insulin exocytosis in mouse pancreatic islets. J. Physiol. (Lond.) 570, 271–282 (2006).
Vaag, A., Henriksen, J.E., Madsbad, S., Holm, N. & Beck-Nielsen, H. Insulin secretion, insulin action, and hepatic glucose production in identical twins discordant for non-insulin-dependent diabetes mellitus. J. Clin. Invest. 95, 690–698 (1995).
Ashcroft, F.M. & Rorsman, P. Electrophysiology of the pancreatic beta-cell. Prog. Biophys. Mol. Biol. 54, 87–143 (1989).
Landa, L.R. Jr. et al. Interplay of Ca2+ and cAMP signaling in the insulin-secreting MIN6 beta-cell line. J. Biol. Chem. 280, 31294–31302 (2005).
Maeda, M. et al. Periodic signaling controlled by an oscillatory circuit that includes protein kinases ERK2 and PKA. Science 304, 875–878 (2004).
Hilioti, Z. et al. Oscillatory phosphorylation of yeast Fus3 MAP kinase controls periodic gene expression and morphogenesis. Curr. Biol. 18, 1700–1706 (2008).
Zhang, J., Ma, Y., Taylor, S.S. & Tsien, R.Y. Genetically encoded reporters of protein kinase A activity reveal impact of substrate tethering. Proc. Natl. Acad. Sci. USA 98, 14997–15002 (2001).
Zhang, J., Hupfeld, C.J., Taylor, S.S., Olefsky, J.M. & Tsien, R.Y. Insulin disrupts beta-adrenergic signalling to protein kinase A in adipocytes. Nature 437, 569–573 (2005).
Grynkiewicz, G., Poenie, M. & Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260, 3440–3450 (1985).
Leiser, M. & Fleischer, N. cAMP-dependent phosphorylation of the cardiac-type alpha 1 subunit of the voltage-dependent Ca2+ channel in a murine pancreatic beta-cell line. Diabetes 45, 1412–1418 (1996).
Bugrim, A.E. Regulation of Ca2+ release by cAMP-dependent protein kinase. A mechanism for agonist-specific calcium signaling? Cell Calcium 25, 219–226 (1999).
Allen, M.D. & Zhang, J. Subcellular dynamics of protein kinase A activity visualized by FRET-based reporters. Biochem. Biophys. Res. Commun. 348, 716–721 (2006).
DiPilato, L.M., Cheng, X. & Zhang, J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc. Natl. Acad. Sci. USA 101, 16513–16518 (2004).
Violin, J.D. et al. beta2-adrenergic receptor signaling and desensitization elucidated by quantitative modeling of real time cAMP dynamics. J. Biol. Chem. 283, 2949–2961 (2008).
Chay, T.R. & Keizer, J. Minimal model for membrane oscillations in the pancreatic beta-cell. Biophys. J. 42, 181–190 (1983).
Oliveria, S.F., Dell'Acqua, M.L. & Sather, W.A. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron 55, 261–275 (2007).
Bergsten, P., Grapengiesser, E., Gylfe, E., Tengholm, A. & Hellman, B. Synchronous oscillations of cytoplasmic Ca2+ and insulin release in glucose-stimulated pancreatic islets. J. Biol. Chem. 269, 8749–8753 (1994).
Dyachok, O., Isakov, Y., Sagetorp, J. & Tengholm, A. Oscillations of cyclic AMP in hormone-stimulated insulin-secreting beta-cells. Nature 439, 349–352 (2006).
Lester, L.B., Faux, M.C., Nauert, J.B. & Scott, J.D. Targeted protein kinase A and PP-2B regulate insulin secretion through reversible phosphorylation. Endocrinology 142, 1218–1227 (2001).
Neves, S.R. et al. Cell shape and negative links in regulatory motifs together control spatial information flow in signaling networks. Cell 133, 666–680 (2008).
Woods, N.M., Cuthbertson, K.S.R. & Cobbold, P.H. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature 319, 600–602 (1986).
Berridge, M.J. & Galione, A. Cytosolic calcium oscillators. FASEB J. 2, 3074–3082 (1988).
Putney, J.W. & Bird, G.S. Cytoplasmic calcium oscillations and store-operated calcium influx. J. Physiol. (Lond.) 586, 3055–3059 (2008).
Li, W., Llopis, J., Whitney, M., Zlokarnik, G. & Tsien, R.Y. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature 392, 936–941 (1998).
Dolmetsch, R.E., Xu, K. & Lewis, R.S. Calcium oscillations increase the efficacy and specificity of calcium-dependent gene expression. Nature 392, 933–936 (1998).
Dunn, T.A. et al. Imaging of cAMP levels and protein kinase A activity reveals that retinal waves drive oscillations in second-messenger cascades. J. Neurosci. 26, 12807–12815 (2006).
Violin, J.D., Zhang, J., Tsien, R.Y. & Newton, A.C. A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase C. J. Cell Biol. 161, 899–909 (2003).
Shankaran, H. et al. Rapid and sustained nuclear-cytoplasmic ERK oscillations induced by epidermal growth factor. Mol. Syst. Biol. 5, 332 (2009).
Markoulaki, S., Matson, S. & Ducibella, T. Fertilization stimulates long-lasting oscillations of CaMKII activity in mouse eggs. Dev. Biol. 272, 15–25 (2004).
Zaccolo, M. & Pozzan, T. CAMP and Ca2+ interplay: a matter of oscillation patterns. Trends Neurosci. 26, 53–55 (2003).
Borodinsky, L.N. & Spitzer, N.C. Second messenger pas de deux: the coordinated dance between calcium and cAMP. Sci. STKE 2006, pe22 (2006).
Allen, M.D. & Zhang, J. A tunable FRET circuit for engineering fluorescent biosensors. Angew. Chem. Int. Edn Engl. 47, 500–502 (2008).
Acknowledgements
We thank J.-I. Miyazaki (Osaka University), G.G. Holz (SUNY Upstate Medical University) and G.H. Hart (Johns Hopkins University) for providing cell lines. We also thank X. Li (Johns Hopkins University) for initial technical assistance. This work was supported by US National Institutes of Health grants R01 DK073368 and DP1OD006419 (to J.Z.) and GM072024 and RR020839 (to A.L.).
Author information
Authors and Affiliations
Contributions
Q.N. and J.Z. conceived the experimental aspect of the project and did the initial experiments; A.L. designed the modeling aspect of the project. Q.N. and N.-N.A.-H. performed the majority of the experiments. A.G. constructed the mathematical model and performed the simulations. X.G. performed the western analyses. M.D.A. designed and generated one of the biosensors. J.Z., Q.N. and A.L. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Figures 1–18 (PDF 11906 kb)
Rights and permissions
About this article
Cite this article
Ni, Q., Ganesan, A., Aye-Han, NN. et al. Signaling diversity of PKA achieved via a Ca2+-cAMP-PKA oscillatory circuit. Nat Chem Biol 7, 34–40 (2011). https://doi.org/10.1038/nchembio.478
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.478
This article is cited by
-
Protective effects of curcumin on desipramine-induced islet β-cell damage via AKAP150/PKA/PP2B complex
Acta Pharmacologica Sinica (2024)
-
Spectrally filtered passive Si photodiode array for on-chip fluorescence imaging of intracellular calcium dynamics
Scientific Reports (2019)
-
Optical tools for understanding the complexity of β-cell signalling and insulin release
Nature Reviews Endocrinology (2018)
-
Epicatechin potentiation of glucose-stimulated insulin secretion in INS-1 cells is not dependent on its antioxidant activity
Acta Pharmacologica Sinica (2018)
-
Molecular Imaging in Synthetic Biology, and Synthetic Biology in Molecular Imaging
Molecular Imaging and Biology (2017)