Abstract
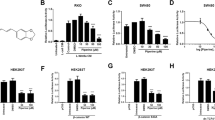
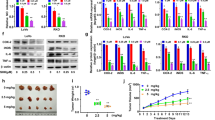
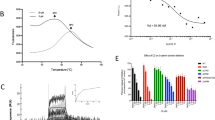
Wnt/β-catenin signaling is critically involved in metazoan development, stem cell maintenance and human disease. Using Xenopus laevis egg extract to screen for compounds that both stabilize Axin and promote β-catenin turnover, we identified an FDA-approved drug, pyrvinium, as a potent inhibitor of Wnt signaling (EC50 of ∼10 nM). We show pyrvinium binds all casein kinase 1 (CK1) family members in vitro at low nanomolar concentrations and pyrvinium selectively potentiates casein kinase 1α (CK1α) kinase activity. CK1α knockdown abrogates the effects of pyrvinium on the Wnt pathway. In addition to its effects on Axin and β-catenin levels, pyrvinium promotes degradation of Pygopus, a Wnt transcriptional component. Pyrvinium treatment of colon cancer cells with mutation of the gene for adenomatous polyposis coli (APC) or β-catenin inhibits both Wnt signaling and proliferation. Our findings reveal allosteric activation of CK1α as an effective mechanism to inhibit Wnt signaling and highlight a new strategy for targeted therapeutics directed against the Wnt pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Yamamoto, H. et al. Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3beta regulates its stability. J. Biol. Chem. 274, 10681–10684 (1999).
Tolwinski, N.S. et al. Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3beta activity. Dev. Cell 4, 407–418 (2003).
Kofron, M. et al. Wnt11/beta-catenin signaling in both oocytes and early embryos acts through LRP6-mediated regulation of axin. Development 134, 503–513 (2007).
Cselenyi, C.S. et al. LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3's phosphorylation of beta-catenin. Proc. Natl. Acad. Sci. USA 105, 8032–8037 (2008).
Barker, N. & Clevers, H. Mining the Wnt pathway for cancer therapeutics. Nat. Rev. Drug Discov. 5, 997–1014 (2006).
Kinzler, K.W. et al. Identification of FAP locus genes from chromosome 5q21. Science 253, 661–665 (1991).
Klaus, A. & Birchmeier, W. Wnt signalling and its impact on development and cancer. Nat. Rev. Cancer 8, 387–398 (2008).
Salic, A., Lee, E., Mayer, L. & Kirschner, M.W. Control of beta-catenin stability: reconstitution of the cytoplasmic steps of the wnt pathway in Xenopus egg extracts. Mol. Cell 5, 523–532 (2000).
Hempelmann, E. Hemozoin biocrystallization in Plasmodium falciparum and the antimalarial activity of crystallization inhibitors. Parasitol. Res. 100, 671–676 (2007).
Downey, A.S., Chong, C.R., Graczyk, T.K. & Sullivan, D.J. Efficacy of pyrvinium pamoate against Cryptosporidium parvum infection in vitro and in a neonatal mouse model. Antimicrob. Agents Chemother. 52, 3106–3112 (2008).
Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell 127, 469–480 (2006).
Xu, Q. et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 116, 883–895 (2004).
Lustig, B. et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell. Biol. 22, 1184–1193 (2002).
Jho, E.H. et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell. Biol. 22, 1172–1183 (2002).
de la Roche, M., Worm, J. & Bienz, M. The function of BCL9 in Wnt/beta-catenin signaling and colorectal cancer cells. BMC Cancer 8, 199 (2008).
He, T.C. et al. Identification of c-MYC as a target of the APC pathway. Science 281, 1509–1512 (1998).
Miyamoto, D.T., Perlman, Z.E., Burbank, K.S., Groen, A.C. & Mitchison, T.J. The kinesin Eg5 drives poleward microtubule flux in Xenopus laevis egg extract spindles. J. Cell Biol. 167, 813–818 (2004).
Larabell, C.A. et al. Establishment of the dorso-ventral axis in Xenopus embryos is presaged by early asymmetries in beta-catenin that are modulated by the Wnt signaling pathway. J. Cell Biol. 136, 1123–1136 (1997).
De Robertis, E.M. & Kuroda, H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu. Rev. Cell Dev. Biol. 20, 285–308 (2004).
Bhanot, P. et al. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 382, 225–230 (1996).
Whangbo, J. & Kenyon, C. A Wnt signaling system that specifies two patterns of cell migration in C. elegans. Mol. Cell 4, 851–858 (1999).
Gleason, J.E., Korswagen, H.C. & Eisenmann, D.M. Activation of Wnt signaling bypasses the requirement for RTK/Ras signaling during C. elegans vulval induction. Genes Dev. 16, 1281–1290 (2002).
Korswagen, H.C. et al. The Axin-like protein PRY-1 is a negative regulator of a canonical Wnt pathway in C. elegans. Genes Dev. 16, 1291–1302 (2002).
Lewis, J.A., Wu, C.H., Berg, H. & Levine, J.H. The genetics of levamisole resistance in the nematode Caenorhabditis elegans. Genetics 95, 905–928 (1980).
Liu, C. et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108, 837–847 (2002).
Gao, Z.H., Seeling, J.M., Hill, V., Yochum, A. & Virshup, D.M. Casein kinase I phosphorylates and destabilizes the beta-catenin degradation complex. Proc. Natl. Acad. Sci. USA 99, 1182–1187 (2002).
Pierre, M. & Nunez, J. Multisite phosphorylation of tau proteins from rat brain. Biochem. Biophys. Res. Commun. 115, 212–219 (1983).
Knight, Z.A. & Shokat, K.M. Features of selective kinase inhibitors. Chem. Biol. 12, 621–637 (2005).
Price, M.A. CKI, there's more than one: casein kinase I family members in Wnt and Hedgehog signaling. Genes Dev. 20, 399–410 (2006).
Bidère, N. et al. Casein kinase 1alpha governs antigen-receptor-induced NF-kappaB activation and human lymphoma cell survival. Nature 458, 92–96 (2009).
Huang, S.M. et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461, 614–620 (2009).
Chen, B. et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 5, 100–107 (2009).
Sparks, A.B., Morin, P.J., Vogelstein, B. & Kinzler, K.W. Mutational analysis of the APC/beta-catenin/Tcf pathway in colorectal cancer. Cancer Res. 58, 1130–1134 (1998).
Polakis, P. Wnt signaling and cancer. Genes Dev. 14, 1837–1851 (2000).
Aoki, M., Hecht, A., Kruse, U., Kemler, R. & Vogt, P.K. Nuclear endpoint of Wnt signaling: neoplastic transformation induced by transactivating lymphoid-enhancing factor 1. Proc. Natl. Acad. Sci. USA 96, 139–144 (1999).
Faux, M.C. et al. Restoration of full-length adenomatous polyposis coli (APC) protein in a colon cancer cell line enhances cell adhesion. J. Cell Sci. 117, 427–439 (2004).
Hämmerlein, A., Weiske, J. & Huber, O. A second protein kinase CK1-mediated step negatively regulates Wnt signalling by disrupting the lymphocyte enhancer factor-1/beta-catenin complex. Cell. Mol. Life Sci. 62, 606–618 (2005).
Kramps, T. et al. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell 109, 47–60 (2002).
Thompson, B., Townsley, F., Rosin-Arbesfeld, R., Musisi, H. & Bienz, M. A new nuclear component of the Wnt signalling pathway. Nat. Cell Biol. 4, 367–373 (2002).
Parker, D.S., Jemison, J. & Cadigan, K.M. Pygopus, a nuclear PHD-finger protein required for Wingless signaling in Drosophila. Development 129, 2565–2576 (2002).
Knippschild, U. et al. The casein kinase 1 family: participation in multiple cellular processes in eukaryotes. Cell. Signal. 17, 675–689 (2005).
Esumi, H., Lu, J., Kurashima, Y. & Hanaoka, T. Antitumor activity of pyrvinium pamoate, 6-(dimethylamino)-2-[2-(2,5-dimethyl-1-phenyl-1H-pyrrol-3-yl)ethenyl]-1-me thyl-quinolinium pamoate salt, showing preferential cytotoxicity during glucose starvation. Cancer Sci. 95, 685–690 (2004).
Rosenbluth, J.M., Mays, D.J., Pino, M.F., Tang, L.J. & Pietenpol, J.A. A gene signature-based approach identifies mTOR as a regulator of p73. Mol. Cell. Biol. 28, 5951–5964 (2008).
Inoki, K. et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126, 955–968 (2006).
Yu, D.H. et al. Pyrvinium targets the unfolded protein response to hypoglycemia and its anti-tumor activity is enhanced by combination therapy. PLoS ONE 3, e3951 (2008).
Jones, J.O. et al. Non-competitive androgen receptor inhibition in vitro and in vivo. Proc. Natl. Acad. Sci. USA 106, 7233–7238 (2009).
Matschinsky, F.M. Assessing the potential of glucokinase activators in diabetes therapy. Nat. Rev. Drug Discov. 8, 399–416 (2009).
Mithani, S.K. et al. Smad3 has a critical role in TGF-beta-mediated growth inhibition and apoptosis in colonic epithelial cells. J. Surg. Res. 117, 296–305 (2004).
Gille, H. et al. ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO J. 14, 951–962 (1995).
Goenka, S. & Boothby, M. Selective potentiation of Stat-dependent gene expression by collaborator of Stat6 (CoaSt6), a transcriptional cofactor. Proc. Natl. Acad. Sci. USA 103, 4210–4215 (2006).
Acknowledgements
We thank members of the Lee laboratory, K. Gould, V. Siegel and R. Roberts-Galbraith for critical reading of the manuscript; J. Merkle for help with Drosophila S2 cell culture; members of the Institute of Chemistry and Cell Biology–Longwood for assistance with screening; M. Beinz, K. Basler, J. Nathans, R. Nusse, S. Hiebert, R. Coffey, B. Gumbiner and M. Lenardo for reagents. Nematode strains were obtained from the C. elegans Genetics Center, which is supported by the US National Institutes of Health National Center for Research Resources. This work was supported by American Cancer Society Research Scholar Grant RSG-05-126-01, American Cancer Society Institutional Research Grant IRG-58-009-46, National Cancer Institute Grant GI SPORE P50 CA95103, Mouse Models of Human Cancers Consortium (US National Institutes of Health–National Cancer Institute) 5U01 CA084239, US National Institutes of Health Grant 1 R01 GM081635-01 (E.L.); US National Institutes of Health Grant 1 R01 NS26115 (D.M.M.); American Heart Association Predoctoral Fellowship 0615279B, Molecular Endocrinology Training Grant 5 T 32 DK007563, Training Program in Developmental Biology 5 T32 HD007502 (National Institute of Child Health and Human Development) (C.A.T.); US National Institute of General Medical Studies Medical-Scientist Training Grant 5 T32 GM007347 (C.S.C. and A.J.H.); American Heart Association Predoctoral Fellowships 0615162B, US National Institutes of Health Cancer Biology Training Grant T32 CA09592 (K.K.J.). E.L. is a recipient of a Pew Scholarship in the Biomedical Sciences.
Author information
Authors and Affiliations
Contributions
C.A.T. designed, performed and analyzed biochemical, extract and cell culture experiments. C.A.T. and E.T. performed screen under E.L. and A.S.'s guidance. J.S. designed and performed C. elegans experiments under D.M.M.'s guidance. E.T. and A.J.H. performed Xenopus embryo experiments. B.L. provided bioinformatics support. D.O., A.G.W., K.K., B.M., V.P.G. and G.A.S. designed and performed chemical synthesis. C.S.C., K.K.J., A.J.H., B.I.H. and L.A.L. provided essential reagents and discussions. K.C.M. provided technical assistance. C.A.T., E.L. and L.A.L. wrote the manuscript with advice from all authors. E.L. guided all aspects of study.
Corresponding author
Ethics declarations
Competing interests
Ethan Lee is cofounder of StemSynergy Therapeutics Inc., a company that aims to develop inhibitors of major signaling pathways (including the Wnt pathway) as potential chemotherapeutic agents. Darren Orton is an employee of StemSynergy Therapeutics Inc.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Results (PDF 4840 kb)
Rights and permissions
About this article
Cite this article
Thorne, C., Hanson, A., Schneider, J. et al. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1α. Nat Chem Biol 6, 829–836 (2010). https://doi.org/10.1038/nchembio.453
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.453
This article is cited by
-
Ablation of Wnt signaling in bone marrow stromal cells overcomes microenvironment-mediated drug resistance in acute myeloid leukemia
Scientific Reports (2024)
-
PGF2alpha Inhibits 20alpha-HSD Expression by Suppressing CK1alpha-induced ERK and SP1 Activation in the Corpus Luteum of Pregnant Mice
Reproductive Sciences (2024)
-
The strategies to cure cancer patients by eradicating cancer stem-like cells
Molecular Cancer (2023)
-
Unravelling morphoea aetiopathogenesis by next-generation sequencing of paired skin biopsies
Archives of Dermatological Research (2023)
-
Molecular targets and therapeutic strategies for triple-negative breast cancer
Molecular Biology Reports (2023)