Abstract
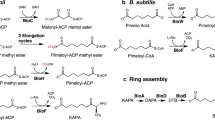
Although biotin is an essential enzyme cofactor found in all three domains of life, our knowledge of its biosynthesis remains fragmentary. Most of the carbon atoms of biotin are derived from pimelic acid, a seven-carbon dicarboxylic acid, but the mechanism whereby this intermediate is assembled remains unknown. Genetic analysis in Escherichia coli identified only two genes of unknown function required for pimelate synthesis, bioC and bioH. We report in vivo and in vitro evidence that the pimeloyl moiety is synthesized by a modified fatty acid synthetic pathway in which the ω-carboxyl group of a malonyl-thioester is methylated by BioC, which allows recognition of this atypical substrate by the fatty acid synthetic enzymes. The malonyl-thioester methyl ester enters fatty acid synthesis as the primer and undergoes two reiterations of the fatty acid elongation cycle to give pimeloyl-acyl carrier protein (ACP) methyl ester, which is hydrolyzed to pimeloyl-ACP and methanol by BioH.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Marquet, A., Bui, B.T.S. Florentin, D. Biosynthesis of biotin and lipoic acid, Vitamins & Hormones 61 51–101 (Academic Press, 2001).
Webb, M.E., Marquet, A., Mendel, R.R., Rebeille, F. & Smith, A.G. Elucidating biosynthetic pathways for vitamins and cofactors. Nat. Prod. Rep. 24, 988–1008 (2007).
Ifuku, O. et al. Origin of the carbon atoms of biotin. Eur. J. Biochem. 220, 585–591 (1994).
Sanyal, I., Lee, S.-L. & Flint, D.H. Biosynthesis of pimeloyl-CoA, a biotin precursor in Escherichia coli, follows a modified fatty acid synthesis pathway: 13C-labeling studies. J. Am. Chem. Soc. 116, 2637–2638 (1994).
Cleary, P.P. & Campbell, A. Deletion and complementation analysis of the biotin gene cluster of Escherichia coli. J. Bacteriol. 112, 830–839 (1972).
Lemoine, Y., Wach, A. & Jeltsch, J.M. To be free or not: the fate of pimelate in Bacillus sphaericus and in Escherichia coli. Mol. Microbiol. 19, 645–647 (1996).
Rolfe, B. & Eisenberg, M.A. Genetic and biochemical analysis of the biotin loci of Escherichia coli K-12. J. Bacteriol. 96, 515–524 (1968).
Kwon, M.A., Kim, H.S., Oh, J.Y., Song, B.K. & Song, J.K. Gene cloning, expression, and characterization of a new carboxylesterase from I sp. SES-01: comparison with IBioHe enzyme. J. Microbiol. Biotechnol. 19, 147–154 (2009).
Sanishvili, R. et al. Integrating structure, bioinformatics, and enzymology to discover function. J. Biol. Chem. 278, 26039–26045 (2003).
Xie, X., Wong, W.W. & Tang, Y. Improving simvastatin bioconversion in Escherichia coli by deletion of bioH. Metab. Eng. 9, 379–386 (2007).
Lezius, A., Ringelmann, E. & Lynen, F. Zur biochemischen Funktion des Biotins. IV. Die Biosynthese des Biotins. Biochem. Z. 336, 510–525 (1963).
Austin, M.B. et al. Crystal structure of a bacterial type III polyketide synthase and enzymatic control of reactive polyketide intermediates. J. Biol. Chem. 279, 45162–45174 (2004).
Tseng, C.C., McLoughlin, S.M., Kelleher, N.L. & Walsh, C.T. Role of the active site cysteine of DpgA, a bacterial type III polyketide synthase. Biochemistry 43, 970–980 (2004).
White, S.W., Zheng, J., Zhang, Y.M. & Rock, C.O. The structural biology of type II fatty acid biosynthesis. Annu. Rev. Biochem. 74, 791–831 (2005).
Chapman-Smith, A. & Cronan, J.E. Jr. The enzymatic biotinylation of proteins: a post-translational modification of exceptional specificity. Trends Biochem. Sci. 24, 359–363 (1999).
Jiang, Y., Chan, C. & Cronan, J.E. The soluble acyl-acyl carrier protein synthetase of Vibrio harveyi B392 is a member of the medium chain Acyl-CoA synthetase family. Biochemistry 45, 10008–10019 (2006).
Jiang, Y., Morgan-Kiss, R.M., Campbell, J.W., Chan, C.H. & Cronan, J.E. Expression of Vibrio harveyi acyl-ACP synthetase allows efficient entry of exogenous fatty acids into the Escherichia coli fatty acid and lipid A synthetic pathways. Biochemistry 49, 718–726 (2010).
Bower, S. et al. Cloning, sequencing, and characterization of the Bacillus subtilis biotin biosynthetic operon. J. Bacteriol. 178, 4122–4130 (1996).
Choi-Rhee, E. & Cronan, J.E. Biotin synthase is catalytic in vivo, but catalysis engenders destruction of the protein. Chem. Biol. 12, 461–468 (2005).
Choi-Rhee, E. & Cronan, J.E. A nucleosidase required for in vivo function of the S-adenosyl-L-methionine radical enzyme, biotin synthase. Chem. Biol. 12, 589–593 (2005).
del Campillo-Campbell, A., Dykhuizen, D. & Cleary, P.P. Enzymic reduction of D-biotin D-sulfoxide to D-biotin. Methods Enzymol. 62, 379–385 (1979).
Lennarz, W.J., Light, R.J. & Bloch, K. A fatty acid synthetase from E. coli. Proc. Natl. Acad. Sci. USA 48, 840–846 (1962).
Campbell, J.W. & Cronan, J.E. Bacterial fatty acid biosynthesis: targets for antibacterial drug discovery. Annu. Rev. Microbiol. 55, 305–332 (2001).
Massengo-Tiassé, R.P. & Cronan, J.E. Vibrio cholerae FabV defines a new class of enoyl-acyl carrier protein reductase. J. Biol. Chem. 283, 1308–1316 (2008).
Pugh, C.S., Borchardt, R.T. & Stone, H.O. Sinefungin, a potent inhibitor of virion mRNA(guanine-7-)-methyltransferase, mRNA(nucleoside-2′-)-methyltransferase, and viral multiplication. J. Biol. Chem. 253, 4075–4077 (1978).
O'Regan, M. et al. Nucleotide sequence of the bioH gene of Escherichia coli. Nucleic Acids Res. 17, 8004 (1989).
Choi, K.H., Heath, R.J. & Rock, C.O. β-Ketoacyl-acyl carrier protein synthase III (FabH) is a determining factor in branched-chain fatty acid biosynthesis. J. Bacteriol. 182, 365–370 (2000).
Tomczyk, N.H. et al. Purification and characterisation of the BIOH protein from the biotin biosynthetic pathway. FEBS Lett. 513, 299–304 (2002).
Cai, H. & Clarke, S. A novel methyltransferase catalyzes the methyl esterification of trans-aconitate in Escherichia coli. J. Biol. Chem. 274, 13470–13479 (1999).
Cai, H., Strouse, J., Dumlao, D., Jung, M.E. & Clarke, S. Distinct reactions catalyzed by bacterial and yeast trans-aconitate methyltransferases. Biochemistry 40, 2210–2219 (2001).
Cronan, J.E. Jr. Molecular properties of short chain acyl thioesters of acyl carrier protein. J. Biol. Chem. 257, 5013–5017 (1982).
Roujeinikova, A. et al. Structural studies of fatty acyl-(acyl carrier protein) thioesters reveal a hydrophobic binding cavity that can expand to fit longer substrates. J. Mol. Biol. 365, 135–145 (2007).
Chan, D.I., Stockner, T., Tieleman, D.P. & Vogel, H.J. Molecular dynamics simulations of the Apo-, Holo-, and acyl-forms of Escherichia coli acyl carrier protein. J. Biol. Chem. 283, 33620–33629 (2008).
Eisenberg, M.A. & Star, C. Synthesis of 7-oxo-8-aminopelargonic acid, a biotin vitamer, in cell-free extracts of Escherichia coli biotin auxotrophs. J. Bacteriol. 96, 1291–1297 (1968).
Cryle, M.J. & Schlichting, I. Structural insights from a P450 carrier protein complex reveal how specificity is achieved in the P450 BioI ACP complex. Proc. Natl. Acad. Sci. USA 105, 15696–15701 (2008).
Stok, J.E. & De Voss, J. Expression, purification, and characterization of BioI: a carbon-carbon bond cleaving cytochrome P450 involved in biotin biosynthesis in Bacillus subtilis. Arch. Biochem. Biophys. 384, 351–360 (2000).
Miller, J. A Short Course in Bacterial Genetics: A Laboratory Manual and Handbook for Escherichia coli and Related Bacteria (Cold Spring Harbor Laboratory Press, 1992).
Rock, C.O. & Cronan, J.E. Jr. Acyl carrier protein from Escherichia coli. Methods Enzymol. 71, 341–351 (1981).
Cronan, J.E. & Thomas, J. Bacterial fatty acid synthesis and its relationships with polyketide synthetic pathways. Methods Enzymol. 459, 395–433 (2009).
Acknowledgements
This work was supported by US National Institutes of Health grant AI15650 from the US National Institute of Allergy and Infectious Diseases.
Author information
Authors and Affiliations
Contributions
S.L., R.E.H. and J.E.C. performed experiments, and S.L. and J.E.C. designed experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Results (PDF 2309 kb)
Rights and permissions
About this article
Cite this article
Lin, S., Hanson, R. & Cronan, J. Biotin synthesis begins by hijacking the fatty acid synthetic pathway. Nat Chem Biol 6, 682–688 (2010). https://doi.org/10.1038/nchembio.420
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.420
This article is cited by
-
Recombinant expression of insoluble enzymes in Escherichia coli: a systematic review of experimental design and its manufacturing implications
Microbial Cell Factories (2021)
-
Initiating polyketide biosynthesis by on-line methyl esterification
Nature Communications (2021)
-
Biochemical and structural characterization of the BioZ enzyme engaged in bacterial biotin synthesis pathway
Nature Communications (2021)
-
α-proteobacteria synthesize biotin precursor pimeloyl-ACP using BioZ 3-ketoacyl-ACP synthase and lysine catabolism
Nature Communications (2020)
-
Mimicking the human environment in mice reveals that inhibiting biotin biosynthesis is effective against antibiotic-resistant pathogens
Nature Microbiology (2019)