Abstract
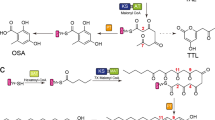
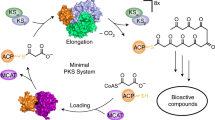
The protein phosphatase inhibitor RK-682 is one of a number of potentially valuable tetronate polyketide natural products. Understanding how the tetronate ring is formed has been frustrated by the inaccessibility of the putative substrates. We report the heterologous expression of rk genes in Saccharopolyspora erythraea and reconstitution of the RK-682 pathway using recombinant enzymes, and we show that RkD is the enzyme required for RK-682 formation from acyl carrier protein–bound substrates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Weissman, K.J. & Leadlay, P.F. Nat. Rev. Microbiol. 3, 925–936 (2005).
Jia, X.Y. et al. Chem. Biol. 13, 575–585 (2006).
Bister, B. et al. Angew. Chem. Int. Edn Engl. 43, 2574–2576 (2004).
Demydchuk, Y. et al. ChemBioChem 9, 1136–1145 (2008).
Zhang, H. et al. J. Am. Chem. Soc. 129, 14670–14683 (2007).
Fang, J. et al. J. Bacteriol. 190, 6014–6025 (2008).
Zhao, P. et al. Org. Biomol. Chem. 7, 1454–1460 (2009).
Hamaguchi, T., Sudo, T. & Osada, H. FEBS Lett. 372, 54–58 (1995).
Fecik, R.A. Nat. Chem. Biol. 3, 531–532 (2007).
Kwon, S.J., Lee, M.Y., Ku, B., Sherman, D.H. & Dordick, J.S. ACS Chem. Biol. 2, 419–425 (2007).
Balibar, C.J., Howard-Jones, A.R. & Walsh, C.T. Nat. Chem. Biol. 3, 584–592 (2007).
Xie, X., Meehan, M.J., Xu, W., Dorrestein, P.C. & Tang, Y. J. Am. Chem. Soc. 131, 8388–8389 (2009).
Sodeoka, M. et al. Chem. Pharm. Bull. (Tokyo) 49, 206–212 (2001).
Roggo, B.E., Hug, P., Moss, S., Raschdorf, F. & Peter, H.H. J. Antibiot. (Tokyo) 47, 143–147 (1994).
Dorrestein, P.C. et al. J. Am. Chem. Soc. 128, 10386–10387 (2006).
Sun, Y., Hong, H., Gillies, F., Spencer, J.B. & Leadlay, P.F. ChemBioChem 9, 150–156 (2008).
Rowe, C.J., Cortés, J., Gaisser, S., Staunton, J. & Leadlay, P.F. Gene 216, 215–223 (1998).
Wilkinson, C.J. et al. J. Mol. Microbiol. Biotechnol. 4, 417–426 (2002).
Butler, A.R., Bate, N. & Cundliffe, E. Chem. Biol. 6, 287–292 (1999).
Heathcote, M.L., Leadlay, P.F. & Staunton, J. Chem. Biol. 8, 207–220 (2001).
Babbitt, P.C. et al. Biochemistry 31, 5594–5604 (1992).
Gaitatzis, N., Hans, A., Müller, R. & Beyer, S. J. Biochem. 129, 119–124 (2001).
Chen, A.Y., Cane, D.E. & Khosla, C. Chem. Biol. 14, 784–792 (2007).
Kwon, H.J. et al. Science 297, 1327–1330 (2002).
Stinear, T.P. et al. Proc. Natl. Acad. Sci. USA 101, 1345–1349 (2004).
Acknowledgements
This work was supported by the Biotechnology and Biological Sciences Research Council through project grant BB/D018943/1 to P.F.L. F.H. was supported by a Deutsche Forschungsgemeinschaft Research Fellowship and a Marie Curie Intra-European Fellowship. M.T. was supported by a fellowship from the Herchel Smith Fund of Cambridge University.
Author information
Authors and Affiliations
Contributions
Y.S., H.O. and P.F.L. formulated the project; Y.S., Y.D. and J.C. carried out cloning and analysis of the gene cluster; Y.S. carried out gene knockouts, heterologous expression and in vitro reconstitution; F.H. carried out chemical synthesis and part of the in vitro reconstitution; M.T. carried out high-resolution mass analysis; Y.S., H.O. and P.F.L. wrote the manuscript.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Results (PDF 6686 kb)
Rights and permissions
About this article
Cite this article
Sun, Y., Hahn, F., Demydchuk, Y. et al. In vitro reconstruction of tetronate RK-682 biosynthesis. Nat Chem Biol 6, 99–101 (2010). https://doi.org/10.1038/nchembio.285
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.285
This article is cited by
-
A dual transacylation mechanism for polyketide synthase chain release in enacyloxin antibiotic biosynthesis
Nature Chemistry (2019)
-
Characterization and heterologous expression of the neoabyssomicin/abyssomicin biosynthetic gene cluster from Streptomyces koyangensis SCSIO 5802
Microbial Cell Factories (2018)
-
Building a bio-based industry in the Middle East through harnessing the potential of the Red Sea biodiversity
Applied Microbiology and Biotechnology (2017)
-
An enzymatic [4+2] cyclization cascade creates the pentacyclic core of pyrroindomycins
Nature Chemical Biology (2015)
-
Biosynthesis of the mycotoxin tenuazonic acid by a fungal NRPS–PKS hybrid enzyme
Nature Communications (2015)