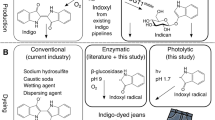
Abstract
Indigo is an ancient dye uniquely capable of producing the signature tones in blue denim; however, the dyeing process requires chemical steps that are environmentally damaging. We describe a sustainable dyeing strategy that not only circumvents the use of toxic reagents for indigo chemical synthesis but also removes the need for a reducing agent for dye solubilization. This strategy utilizes a glucose moiety as a biochemical protecting group to stabilize the reactive indigo precursor indoxyl to form indican, preventing spontaneous oxidation to crystalline indigo during microbial fermentation. Application of a β-glucosidase removes the protecting group from indican, resulting in indigo crystal formation in the cotton fibers. We identified the gene coding for the glucosyltransferase PtUGT1 from the indigo plant Polygonum tinctorium and solved the structure of PtUGT1. Heterologous expression of PtUGT1 in Escherichia coli supported high indican conversion, and biosynthesized indican was used to dye cotton swatches and a garment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Splitstoser, J.C., Dillehay, T.D., Wouters, J. & Claro, A. Early pre-Hispanic use of indigo blue in Peru. Sci. Adv. 2, e1501623 (2016).
Balfour-Paul, J. Indigo (Firefly Books, 2011).
Wolf, L.K. What's That Stuff? Blue Jeans. Chem. Eng. News 89, 44 (2011).
Schimper, C.B., Ibanescu, C. & Bechtold, T. Surface activation of dyed fabric for cellulase treatment. Biotechnol. J. 6, 1280–1285 (2011).
Pfleger, J. Process of making indoxyl derivatives. US Patent 680,395 (1901).
Paul, R. Denim. (Elsevier Ltd., 2015).
Blackburn, R.S., Bechtold, T. & John, P. The development of indigo reduction methods and pre-reduced indigo products. Color. Technol. 125, 193–207 (2009).
Ensley, B.D. et al. Expression of naphthalene oxidation genes in Escherichia coli results in the biosynthesis of indigo. Science 222, 167–169 (1983).
Murdock, D., Ensley, B.D., Serdar, C. & Thalen, M. Construction of metabolic operons catalyzing the de novo biosynthesis of indigo in Escherichia coli. Nat. Biotechnol. 11, 381–386 (1993).
Berry, A., Dodge, T.C., Pepsin, M. & Weyler, W. Application of metabolic engineering to improve both the production and use of biotech indigo. J. Ind. Microbiol. Biotechnol. 28, 127–133 (2002).
Han, G.H. et al. Bio-indigo production in two different fermentation systems using recombinant Escherichia coli cells harboring a flavin-containing monooxygenase gene (fmo). Process Biochem. 46, 788–791 (2011).
Padden, A.N. et al. An indigo-reducing moderate thermophile from a woad vat, Clostridium isatidis sp. nov. Int. J. Syst. Bacteriol. 49, 1025–1031 (1999).
Yumoto, I. et al. Alkalibacterium psychrotolerans sp. nov., a psychrotolerant obligate alkaliphile that reduces an indigo dye. Int. J. Syst. Evol. Microbiol 54, 2379–2383 (2004).
Gäng, M., Krüger, R. & Miederer, P. Concentrated leucoindigo solutions. US Patent 6,428,581 (2002).
Roessler, A., Crettenand, D., Dossenbach, O. & Rys, P. Electrochemical reduction of indigo in fixed and fluidized beds of graphite granules. J. Appl. Electrochem. 33, 901–908 (2003).
Minami, Y., Nishimura, O., Hara-Nishimura, I., Nishimura, M. & Matsubara, H. Tissue and intracellular localization of indican and the purification and characterization of indican synthase from indigo plants. Plant Cell Physiol. 41, 218–225 (2000).
Minami, Y. et al. β-Glucosidase in the indigo plant: intracellular localization and tissue specific expression in leaves. Plant Cell Physiol. 38, 1069–1074 (1997).
Dang, T.-T.T., Chen, X. & Facchini, P.J. Acetylation serves as a protective group in noscapine biosynthesis in opium poppy. Nat. Chem. Biol. 11, 104–106 (2015).
Chen, J. et al. Biosynthesis of the active compounds of Isatis indigotica based on transcriptome sequencing and metabolites profiling. BMC Genomics 14, 857 (2013).
Tang, X. et al. High-throughput sequencing and De Novo assembly of the Isatis indigotica transcriptome. PLoS One 9, e102963–e102968 (2014).
Minami, Y., Sarangi, B.K. & Thul, S.T. Transcriptome analysis for identification of indigo biosynthesis pathway genes in Polygonum tinctorium. Biologia 70, 1026–1032 (2015).
John, P. in Handbook of Natural Colorants (eds. Bechtold, T. & Mussak, R) Ch. 8 (John Wiley and Sons, 2009).
Gilbert, K.G. et al. Quantitative analysis of indigo and indigo precursors in leaves of Isatis spp. and Polygonum tinctorium. Biotechnol. Prog. 20, 1289–1292 (2004).
Grabherr, M.G. et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 29, 644–652 (2011).
Mackenzie, P.I. et al. The UDP glycosyltransferase gene superfamily: recommended nomenclature update based on evolutionary divergence. Pharmacogenetics 7, 255–269 (1997).
Lombard, V., Golaconda Ramulu, H., Drula, E., Coutinho, P.M. & Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 42, D490–D495 (2014).
Lairson, L.L., Henrissat, B., Davies, G.J. & Withers, S.G. Glycosyltransferases: structures, functions, and mechanisms. Annu. Rev. Biochem. 77, 521–555 (2008).
Osmani, S.A., Bak, S. & Møller, B.L. Substrate specificity of plant UDP-dependent glycosyltransferases predicted from crystal structures and homology modeling. Phytochemistry 70, 325–347 (2009).
Wang, X. Structure, mechanism and engineering of plant natural product glycosyltransferases. FEBS Lett. 583, 3303–3309 (2009).
Loutre, C. et al. Isolation of a glucosyltransferase from Arabidopsis thaliana active in the metabolism of the persistent pollutant 3,4-dichloroaniline. Plant J. 34, 485–493 (2003).
Brazier-Hicks, M. et al. Characterization and engineering of the bifunctional N- and O-glucosyltransferase involved in xenobiotic metabolism in plants. Proc. Natl. Acad. Sci. USA 104, 20238–20243 (2007).
Nakamura, C.E. & Whited, G.M. Metabolic engineering for the microbial production of 1,3-propanediol. Curr. Opin. Biotechnol. 14, 454–459 (2003).
Yim, H. et al. Metabolic engineering of Escherichia coli for direct production of 1,4-butanediol. Nat. Chem. Biol. 7, 445–452 (2011).
Patnaik, R. & Liao, J.C. Engineering of Escherichia coli central metabolism for aromatic metabolite production with near theoretical yield. Appl. Environ. Microbiol. 60, 3903–3908 (1994).
Malla, S., Pandey, R.P., Kim, B.-G. & Sohng, J.K. Regiospecific modifications of naringenin for astragalin production in Escherichia coli. Biotechnol. Bioeng. 110, 2525–2535 (2013).
Lim, C.G. et al. Development of a recombinant Escherichia coli strain for overproduction of the plant pigment anthocyanin. Appl. Environ. Microbiol. 81, 6276–6284 (2015).
Choi, H.S. et al. A novel flavin-containing monooxygenase from Methylophaga sp. strain SK1 and its indigo synthesis in Escherichia coli. Biochem. Biophys. Res. Commun. 306, 930–936 (2003).
Anderson, J.C. et al. BglBricks: a flexible standard for biological part assembly. J. Biol. Eng. 4, 1 (2010).
Li, G. & Young, K.D. A cAMP-independent carbohydrate-driven mechanism inhibits tnaA expression and TnaA enzyme activity in Escherichia coli. Microbiology 160, 2079–2088 (2014).
Botsford, J.L. & DeMoss, R.D. Catabolite repression of tryptophanase in Escherichia coli. J. Bacteriol. 105, 303–312 (1971).
Minami, Y., Kanafuji, T. & Miura, K. Purification and characterization of a β-glucosidase from Polygonum tinctorium, which catalyzes preferentially the hydrolysis of indican. Biosci. Biotechnol. Biochem. 60, 147–149 (1996).
Song, J., Imanaka, H., Imamura, K., Kajitani, K. & Nakanishi, K. Development of a highly efficient indigo dyeing method using indican with an immobilized β-glucosidase from Aspergillus niger. J. Biosci. Bioeng. 110, 281–287 (2010).
Kim, J.-Y., Lee, J.-Y., Shin, Y.-S. & Kim, G.-J. Characterization of an indican-hydrolyzing enzyme from Sinorhizobium meliloti. Process Biochem. 45, 892–896 (2010).
Baba, T. et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2, 0008 (2006).
Paavilainen, S., Hellman, J. & Korpela, T. Purification, characterization, gene cloning, and sequencing of a new β-glucosidase from Bacillus circulans subsp. alkalophilus. Appl. Environ. Microbiol. 59, 927–932 (1993).
Hansen, E.H. et al. De novo biosynthesis of vanillin in fission yeast (Schizosaccharomyces pombe) and baker's yeast (Saccharomyces cerevisiae). Appl. Environ. Microbiol. 75, 2765–2774 (2009).
Moehs, C.P., Allen, P.V., Friedman, M. & Belknap, W.R. Cloning and expression of solanidine UDP-glucose glucosyltransferase from potato. Plant J. 11, 227–236 (1997).
Etters, J.N. Advances in indigo dyeing: implications for the dyer, apparel manufacturer and environment. Text. Chem. Color. 27, 17–22 (1995).
Sternberg, D., Vijayakumar, P. & Reese, E.T. β-Glucosidase: microbial production and effect on enzymatic hydrolysis of cellulose. Can. J. Microbiol. 23, 139–147 (1977).
Jäger, S., Brumbauer, A., Fehér, E., Réczey, K. & Kiss, L. Production and characterization of β-glucosidases from different Aspergillus strains. World J. Microbiol. Biotechnol. 17, 455–461 (2001).
Bolger, A.M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Magoč, T. & Salzberg, S.L. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27, 2957–2963 (2011).
Crusoe, M.R. et al. The khmer software package: enabling efficient nucleotide sequence analysis. F1000 Res. 4, 900 (2015).
Schulz, M.H., Zerbino, D.R., Vingron, M. & Birney, E. Oases: robust de novo RNA-seq assembly across the dynamic range of expression levels. Bioinformatics 28, 1086–1092 (2012).
Towns, J. et al. XSEDE: accelerating scientific discovery. Comput. Sci. Eng. 16, 62–74 (2014).
Tabb, D.L., McDonald, W.H. & Yates, J.R., III. DTASelect and Contrast: tools for assembling and comparing protein identifications from shotgun proteomics. J. Proteome Res. 1, 21–26 (2002).
Lee, M.E., DeLoache, W.C., Cervantes, B. & Dueber, J.E. A highly characterized yeast toolkit for modular, multipart assembly. ACS Synth. Biol. 4, 975–986 (2015).
Datsenko, K.A. & Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97, 6640–6645 (2000).
Hoang, T.T., Karkhoff-Schweizer, R.R., Kutchma, A.J. & Schweizer, H.P. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212, 77–86 (1998).
Winter, G. xia2: an expert system for macromolecular crystallography data reduction. J. Appl. Crystallogr. 43, 186–190 (2010).
Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
McCoy, A.J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Afonine, P.V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 68, 352–367 (2012).
Emsley, P., Lohkamp, B., Scott, W.G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Chen, V.B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Salentin, S., Schreiber, S., Haupt, V.J., Adasme, M.F. & Schroeder, M. PLIP: fully automated protein-ligand interaction profiler. Nucleic Acids Res. 43, W443–W447 (2015).
Holm, L. & Rosenström, P. Dali server: conservation mapping in 3D. Nucleic Acids Res. 38, W545–W549 (2010).
Itaya, K. & Ui, M. A new micromethod for the colorimetric determination of inorganic phosphate. Clin. Chim. Acta 14, 361–366 (1966).
McKee, J.R. & Zanger, M. A microscale synthesis of indigo: vat dyeing. J. Chem. Educ. 68, A242 (1991).
Acknowledgements
The authors thank the rest of the Berkeley iGEM 2013 team, C. Somerville, N. Sorek, S. Bauer, N. Harris, D. Savage, B. Sights, S. Wagner, and the Dueber Lab, especially S. Bhakta, D. Stanley, L. Latimer, P. Grewal, and S. Halperin, for valuable discussions and experimental assistance. The UC Berkeley Vincent J. Coates Genomics Sequencing Laboratory and Proteomics/Mass Spectrometry Laboratory provided transcriptome and protein sequencing. The M. Chang laboratory (University of California, Berkeley) provided the base E. coli strain. This work was supported by Bakar Fellows Program (fellowship to J.E.D.), NSF CBET 1605465 (J.E.D.), a generous gift from Levi Strauss & Co. (J.E.D.), the US Department of Defense (fellowship to T.M.H. and Z.N.R.) and Agilent (iGEM undergraduate fellowships). Crystallographic experiments were performed as part of the DOE Joint BioEnergy Institute (http://www.jbei.org) which is supported by the US Department of Energy, Office of Science, Office of Biological and Environmental Research, through contract DE-AC02-05CH11231 between Lawrence Berkeley National Laboratory and the US Department of Energy. We thank the Berkeley Center for Structural Biology beamline staff for technical assistance during data collection. The BCSB is supported in part by the National Institutes of Health, National Institute of General Medical Sciences, and the Howard Hughes Medical Institute. The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under Contract No. DE-AC02-05CH11231.
Author information
Authors and Affiliations
Contributions
T.M.H., Z.N.R., and J.E.D. designed the research. T.M.H., D.H.W., Z.N.R., B.C., and R.L.P. collected data. D.H.W. solved the crystallographic structure. T.M.H., D.H.W., Z.N.R., P.D.A., and J.E.D. analyzed the data. T.M.H., D.H.W., and J.E.D. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare competing financial interests in the form of a pending patent application, US application no. 62/127,778.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–5, Supplementary Figures 1–11 (PDF 15489 kb)
Rights and permissions
About this article
Cite this article
Hsu, T., Welner, D., Russ, Z. et al. Employing a biochemical protecting group for a sustainable indigo dyeing strategy. Nat Chem Biol 14, 256–261 (2018). https://doi.org/10.1038/nchembio.2552
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2552
This article is cited by
-
Chemoenzymatic indican for light-driven denim dyeing
Nature Communications (2024)
-
Synthetic microbe-to-plant communication channels
Nature Communications (2024)
-
Indoles and the advances in their biotechnological production for industrial applications
Systems Microbiology and Biomanufacturing (2024)
-
Indigo production goes green: a review on opportunities and challenges of fermentative production
World Journal of Microbiology and Biotechnology (2024)
-
Production of indigo by recombinant bacteria
Bioresources and Bioprocessing (2023)