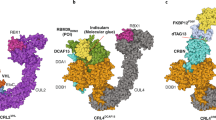
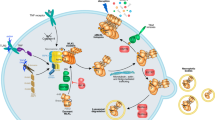
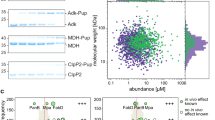
Abstract
Protein degradation plays a central role in many cellular functions. Misfolded and damaged proteins are removed from the cell to avoid toxicity. The concentrations of regulatory proteins are adjusted by degradation at the appropriate time. Both foreign and native proteins are digested into small peptides as part of the adaptive immune response. In eukaryotic cells, an ATP-dependent protease called the proteasome is responsible for much of this proteolysis. Proteins are targeted for proteasomal degradation by a two-part degron, which consists of a proteasome binding signal and a degradation initiation site. Here we describe how both components contribute to the specificity of degradation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Katie Vicari

Katie Vicari

Katie Vicari

Katie Vicari

Katie Vicari
Similar content being viewed by others
Change history
28 October 2009
In the version of this article initially published, a positive competing financial interest statement was noted, where none exists. The error has been corrected in the HTML and PDF versions of the article.
References
Nussbaum, A.K. et al. Cleavage motifs of the yeast 20S proteasome β subunits deduced from digests of enolase 1. Proc. Natl. Acad. Sci. USA 95, 12504–12509 (1998).
Lee, C., Schwartz, M.P., Prakash, S., Iwakura, M. & Matouschek, A. ATP-dependent proteases degrade their substrates by processively unraveling them from the degradation signal. Mol. Cell 7, 627–637 (2001).
Groll, M. et al. Structure of 20S proteasome from yeast at 2.4 Å resolution. Nature 386, 463–471 (1997).
Tanaka, K. The proteasome: overview of structure and functions. Proc. Jpn. Acad., Ser. B, Phys. Biol. Sci. 85, 12–36 (2009).
Finley, D. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu. Rev. Biochem. 78, 477–513 (2009).
Lowe, J. et al. Crystal structure of the 20S proteasome from the archaeon T. acidophilum at 3.4 Å resolution. Science 268, 533–539 (1995).
Groll, M. et al. A gated channel into the proteasome core particle. Nat. Struct. Biol. 7, 1062–1067 (2000).
Johnston, J.A., Johnson, E.S., Waller, P.R.H. & Varshavsky, A. Methotrexate inhibits proteolysis of dihydrofolate reductase by the N-end rule pathway. J. Biol. Chem. 270, 8172–8178 (1995).
Thrower, J.S., Hoffman, L., Rechsteiner, M. & Pickart, C.M. Recognition of the polyubiquitin proteolytic signal. EMBO J. 19, 94–102 (2000).
Weissman, A.M. Themes and variations on ubiquitylation. Nat. Rev. Mol. Cell Biol. 2, 169–178 (2001).
Prakash, S., Tian, L., Ratliff, K.S., Lehotzky, R.E. & Matouschek, A. An unstructured initiation site is required for efficient proteasome-mediated degradation. Nat. Struct. Mol. Biol. 11, 830–837 (2004).
Prakash, S., Inobe, T., Hatch, A.J. & Matouschek, A. Substrate selection by the proteasome during degradation of protein complexes. Nat. Chem. Biol. 5, 29–36 (2009).
Ciechanover, A. & Ben-Saadon, R. N-terminal ubiquitination: more protein substrates join in. Trends Cell Biol. 14, 103–106 (2004).
Cadwell, K. & Coscoy, L. Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase. Science 309, 127–130 (2005).
Hoppe, T. Multiubiquitylation by E4 enzymes: 'one size' doesn't fit all. Trends Biochem. Sci. 30, 183–187 (2005).
Pickart, C.M. & Fushman, D. Polyubiquitin chains: polymeric protein signals. Curr. Opin. Chem. Biol. 8, 610–616 (2004).
Jin, L., Williamson, A., Banerjee, S., Philipp, I. & Rape, M. Mechanism of ubiquitin-chain formation by the human anaphase-promoting complex. Cell 133, 653–665 (2008).
Hofmann, R.M. & Pickart, C.M. In vitro assembly and recognition of Lys-63 polyubiquitin chains. J. Biol. Chem. 276, 27936–27943 (2001).
Saeki, Y. et al. Lysine 63-linked polyubiquitin chain may serve as a targeting signal for the 26S proteasome. EMBO J. 28, 359–371 (2009).
Xu, P. et al. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell 137, 133–145 (2009).
Shaeffer, J.R. & Kania, M.A. Degradation of monoubiquitinated α-globin by 26S proteasomes. Biochemistry 34, 4015–4021 (1995).
Boutet, S.C., Disatnik, M.-H., Chan, L.S., Iori, K. & Rando, T.A. Regulation of Pax3 by proteasomal degradation of monoubiquitinated protein in skeletal muscle progenitors. Cell 130, 349–362 (2007).
Kravtsova-Ivantsiv, Y., Cohen, S. & Ciechanover, A. Modification by single ubiquitin moieties rather than polyubiquitination is sufficient for proteasomal processing of the p105 NF-κB precursor. Mol. Cell 33, 496–504 (2009).
Peng, J. et al. A proteomics approach to understanding protein ubiquitination. Nat. Biotechnol. 21, 921–926 (2003).
Hicke, L. & Riezman, H. Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell 84, 277–287 (1996).
Hicke, L., Schubert, H.L. & Hill, C.P. Ubiquitin-binding domains. Nat. Rev. Mol. Cell Biol. 6, 610–621 (2005).
Elsasser, S. & Finley, D. Delivery of ubiquitinated substrates to protein-unfolding machines. Nat. Cell Biol. 7, 742–749 (2005).
Deveraux, Q., Ustrell, V., Pickart, C. & Rechsteiner, M. A 26 S protease subunit that binds ubiquitin conjugates. J. Biol. Chem. 269, 7059–7061 (1994).
Husnjak, K. et al. Proteasome subunit Rpn13 is a novel ubiquitin receptor. Nature 453, 481–488 (2008).
Lam, Y.A., Lawson, T.G., Velayutham, M., Zweier, J.L. & Pickart, C.M. A proteasomal ATPase subunit recognizes the polyubiquitin degradation signal. Nature 416, 763–767 (2002).
Elsasser, S., Chandler-Militello, D., Muller, B., Hanna, J. & Finley, D. Rad23 and Rpn10 serve as alternative ubiquitin receptors for the proteasome. J. Biol. Chem. 279, 26817–26822 (2004).
Kim, I., Mi, K. & Rao, H. Multiple interactions of rad23 suggest a mechanism for ubiquitylated substrate delivery important in proteolysis. Mol. Biol. Cell 15, 3357–3365 (2004).
Verma, R., Oania, R., Graumann, J. & Deshaies, R.J. Multiubiquitin chain receptors define a layer of substrate selectivity in the ubiquitin-proteasome system. Cell 118, 99–110 (2004).
Chen, L. & Madura, K. Rad23 promotes the targeting of proteolytic substrates to the proteasome. Mol. Cell. Biol. 22, 4902–4913 (2002).
Rao, H. & Sastry, A. Recognition of specific ubiquitin conjugates is important for the proteolytic functions of the ubiquitin-associated domain proteins Dsk2 and Rad23. J. Biol. Chem. 277, 11691–11695 (2002).
Wilkinson, C.R. et al. Proteins containing the UBA domain are able to bind to multi-ubiquitin chains. Nat. Cell Biol. 3, 939–943 (2001).
Hiyama, H. et al. Interaction of hHR23 with S5a. The ubiquitin-like domain of hHR23 mediates interaction with S5a subunit of 26 S proteasome. J. Biol. Chem. 274, 28019–28025 (1999).
Schauber, C. et al. Rad23 links DNA repair to the ubiquitin/proteasome pathway. Nature 391, 715–718 (1998).
Richly, H. et al. A series of ubiquitin binding factors connects CDC48/p97 to substrate multiubiquitylation and proteasomal targeting. Cell 120, 73–84 (2005).
Madura, K. Rad23 and Rpn10: perennial wallflowers join the melee. Trends Biochem. Sci. 29, 637–640 (2004).
Sakata, E. et al. Parkin binds the Rpn10 subunit of 26S proteasomes through its ubiquitin-like domain. EMBO Rep. 4, 301–306 (2003).
Kaplun, L. et al. The DNA damage-inducible UbL-UbA protein Ddi1 participates in Mec1-mediated degradation of Ho endonuclease. Mol. Cell. Biol. 25, 5355–5362 (2005).
Amerik, A.Y. & Hochstrasser, M. Mechanism and function of deubiquitinating enzymes. Biochim. Biophys. Acta 1695, 189–207 (2004).
Verma, R. et al. Role of Rpn11 metalloprotease in deubiquitination and degradation by the 26S proteasome. Science 298, 611–615 (2002).
Yao, T. & Cohen, R.E. A cryptic protease couples deubiquitination and degradation by the proteasome. Nature 419, 403–407 (2002).
Hanna, J. et al. Deubiquitinating enzyme Ubp6 functions noncatalytically to delay proteasomal degradation. Cell 127, 99–111 (2006).
Lam, Y.A., Xu, W., DeMartino, G.N. & Cohen, R.E. Editing of ubiquitin conjugates by an isopeptidase in the 26S proteasome. Nature 385, 737–740 (1997).
Crosas, B. et al. Ubiquitin chains are remodeled at the proteasome by opposing ubiquitin ligase and deubiquitinating activities. Cell 127, 1401–1413 (2006).
Kraut, D.A., Prakash, S. & Matouschek, A. To degrade or release: ubiquitin-chain remodeling. Trends Cell Biol. 17, 419–421 (2007).
Peters, J.M. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol. Cell 9, 931–943 (2002).
Rape, M., Reddy, S.K. & Kirschner, M.W. The processivity of multiubiquitination by the APC determines the order of substrate degradation. Cell 124, 89–103 (2006).
Ye, Y. Diverse functions with a common regulator: ubiquitin takes command of an AAA ATPase. J. Struct. Biol. 156, 29–40 (2006).
Jentsch, S. & Rumpf, S. Cdc48 (p97): a “molecular gearbox” in the ubiquitin pathway? Trends Biochem. Sci. 32, 6–11 (2007).
Jarosch, E. et al. Protein dislocation from the ER requires polyubiquitination and the AAA-ATPase Cdc48. Nat. Cell Biol. 4, 134–139 (2002).
Ye, Y., Meyer, H.H. & Rapoport, T.A. The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature 414, 652–656 (2001).
Bachmair, A. & Varshavsky, A. The degradation signal in a short-lived protein. Cell 56, 1019–1032 (1989).
Petroski, M.D. & Deshaies, R.J. Context of multiubiquitin chain attachment influences the rate of Sic1 degradation. Mol. Cell 11, 1435–1444 (2003).
Takeuchi, J., Chen, H. & Coffino, P. Proteasome substrate degradation requires association plus extended peptide. EMBO J. 26, 123–131 (2007).
Liu, C.W., Corboy, M.J., DeMartino, G.N. & Thomas, P.J. Endoproteolytic activity of the proteasome. Science 299, 408–411 (2003).
Piwko, W. & Jentsch, S. Proteasome-mediated protein processing by bidirectional degradation initiated from an internal site. Nat. Struct. Mol. Biol. 13, 691–697 (2006).
Hinnerwisch, J., Fenton, W.A., Furtak, K.J., Farr, G.W. & Horwich, A.L. Loops in the central channel of ClpA chaperone mediate protein binding, unfolding, and translocation. Cell 121, 1029–1041 (2005).
Martin, A., Baker, T.A. & Sauer, R.T. Pore loops of the AAA+ ClpX machine grip substrates to drive translocation and unfolding. Nat. Struct. Mol. Biol. 15, 1147–1151 (2008).
Park, E. et al. Role of the GYVG pore motif of HslU ATPase in protein unfolding and translocation for degradation by HslV peptidase. J. Biol. Chem. 280, 22892–22898 (2005).
Yamada-Inagawa, T., Okuno, T., Karata, K., Yamanaka, K. & Ogura, T. Conserved pore residues in the AAA protease FtsH are important for proteolysis and its coupling to ATP hydrolysis. J. Biol. Chem. 278, 50182–50187 (2003).
Wang, J. et al. Nucleotide-dependent conformational changes in a protease-associated ATPase HsIU. Structure 9, 1107–1116 (2001).
Wang, J. et al. Crystal structures of the HslVU peptidase-ATPase complex reveal an ATP-dependent proteolysis mechanism. Structure 9, 177–184 (2001).
Heessen, S., Masucci, M.G. & Dantuma, N.P. The UBA2 domain functions as an intrinsic stabilization signal that protects Rad23 from proteasomal degradation. Mol. Cell 18, 225–235 (2005).
Verhoef, L.G. et al. Minimal length requirement for proteasomal degradation of ubiquitin-dependent substrates. FASEB J. 23, 123–133 (2009).
Tian, L., Holmgren, R.A. & Matouschek, A. A conserved processing mechanism regulates the activity of transcription factors Cubitus interruptus and NF-κB. Nat. Struct. Mol. Biol. 12, 1045–1053 (2005).
Johnson, E.S., Gonda, D.K. & Varshavsky, A. Cis-trans recognition and subunit-specific degradation of short-lived proteins. Nature 346, 287–291 (1990).
Hochstrasser, M. & Varshavsky, A. In vivo degradation of a transcriptional regulator: the yeast α2 repressor. Cell 61, 697–708 (1990).
King, R.W., Deshaies, R.J., Peters, J.M. & Kirschner, M.W. How proteolysis drives the cell cycle. Science 274, 1652–1659 (1996).
Nishiyama, A. et al. A nonproteolytic function of the proteasome is required for the dissociation of Cdc2 and cyclin B at the end of M phase. Genes Dev. 14, 2344–2357 (2000).
Jeffrey, P.D. et al. Mechanism of CDK activation revealed by the structure of a cyclin A-CDK2 complex. Nature 376, 313–320 (1995).
King, R.W., Glotzer, M. & Kirschner, M.W. Mutagenic analysis of the destruction signal of mitotic cyclins and structural characterization of ubiquitinated intermediates. Mol. Biol. Cell 7, 1343–1357 (1996).
Klotzbucher, A., Stewart, E., Harrison, D. & Hunt, T. The 'destruction box' of cyclin A allows B-type cyclins to be ubiquitinated, but not efficiently destroyed. EMBO J. 15, 3053–3064 (1996).
Verma, R., McDonald, H., Yates, J.R. III & Deshaies, R.J. Selective degradation of ubiquitinated Sic1 by purified 26S proteasome yields active S phase cyclin-Cdk. Mol. Cell 8, 439–448 (2001).
Dang, Y., Siew, L.M. & Zheng, Y.H. APOBEC3G is degraded by the proteasomal pathway in a Vif-dependent manner without being polyubiquitylated. J. Biol. Chem. 283, 13124–13131 (2008).
Gonzalez, S.L., Stremlau, M., He, X., Basile, J.R. & Munger, K. Degradation of the retinoblastoma tumor suppressor by the human papillomavirus type 16 E7 oncoprotein is important for functional inactivation and is separable from proteasomal degradation of E7. J. Virol. 75, 7583–7591 (2001).
Berezutskaya, E. & Bagchi, S. The human papillomavirus E7 oncoprotein functionally interacts with the S4 subunit of the 26 S proteasome. J. Biol. Chem. 272, 30135–30140 (1997).
Hoyt, M.A. & Coffino, P. Ubiquitin-free routes into the proteasome. Cell. Mol. Life Sci. 61, 1596–1600 (2004).
Jariel-Encontre, I., Bossis, G. & Piechaczyk, M. Ubiquitin-independent degradation of proteins by the proteasome. Biochim. Biophys. Acta 1786, 153–177 (2008).
Orlowski, M. & Wilk, S. Ubiquitin-independent proteolytic functions of the proteasome. Arch. Biochem. Biophys. 415, 1–5 (2003).
Verma, R. & Deshaies, R.J. A proteasome howdunit: the case of the missing signal. Cell 101, 341–344 (2000).
Murakami, Y. et al. Ornithine decarboxylase is degraded by the 26S proteasome without ubiquitination. Nature 360, 597–599 (1992).
Zhang, M., Pickart, C.M. & Coffino, P. Determinants of proteasome recognition of ornithine decarboxylase, a ubiquitin-independent substrate. EMBO J. 22, 1488–1496 (2003).
Zhang, M., MacDonald, A.I., Hoyt, M.A. & Coffino, P. Proteasomes begin ornithine decarboxylase digestion at the C terminus. J. Biol. Chem. 279, 20959–20965 (2004).
Baugh, J.M., Viktorova, E.G. & Pilipenko, E.V. Proteasomes can degrade a significant proportion of cellular proteins independent of ubiquitination. J. Mol. Biol. 386, 814–827 (2009).
Baker, T.A. & Sauer, R.T. ATP-dependent proteases of bacteria: recognition logic and operating principles. Trends Biochem. Sci. 31, 647–653 (2006).
Sauer, R.T. et al. Sculpting the proteome with AAA(+) proteases and disassembly machines. Cell 119, 9–18 (2004).
Striebel, F., Kress, W. & Weber-Ban, E. Controlled destruction: AAA+ ATPases in protein degradation from bacteria to eukaryotes. Curr. Opin. Struct. Biol. 19, 209–217 (2009).
Pearce, M.J., Mintseris, J., Ferreyra, J., Gygi, S.P. & Darwin, K.H. Ubiquitin-like protein involved in the proteasome pathway of Mycobacterium tuberculosis. Science 322, 1104–1107 (2008).
Burns, K.E., Liu, W.T., Boshoff, H.I., Dorrestein, P.C. & Barry, C.E. III. Proteasomal protein degradation in Mycobacteria is dependent upon a prokaryotic ubiquitin-like protein. J. Biol. Chem. 284, 3069–3075 (2009).
Iyer, L., Burroughs, A.M. & Aravind, L. Unraveling the biochemistry and provenance of pupylation: a prokaryotic analog of ubiquitination. Biol. Direct 3, 45 (2008).
Flynn, J.M., Neher, S.B., Kim, Y.I., Sauer, R.T. & Baker, T.A. Proteomic discovery of cellular substrates of the ClpXP protease reveals five classes of ClpX-recognition signals. Mol. Cell 11, 671–683 (2003).
Keiler, K.C., Waller, P.R. & Sauer, R.T. Role of a peptide tagging system in degradation of proteins synthesized from damaged messenger RNA. Science 271, 990–993 (1996).
Hoskins, J.R. & Wickner, S. Two peptide sequences can function cooperatively to facilitate binding and unfolding by ClpA and degradation by ClpAP. Proc. Natl. Acad. Sci. USA 103, 909–914 (2006).
Ninnis, R.L., Spall, S.K., Talbo, G.H., Truscott, K.N. & Dougan, D.A. Modification of PATase by L/F-transferase generates a ClpS-dependent N-end rule substrate in Escherichia coli. EMBO J. 28, 1732–1744 (2009).
Levchenko, I., Seidel, M., Sauer, R.T. & Baker, T.A.A. Specificity-enhancing factor for the ClpXP degradation machine. Science 289, 2354–2356 (2000).
Dougan, D.A., Weber-Ban, E. & Bukau, B. Targeted delivery of an ssrA-tagged substrate by the adaptor protein SspB to its cognate AAA+ protein ClpX. Mol. Cell 12, 373–380 (2003).
Muffler, A., Fischer, D., Altuvia, S., Storz, G. & Hengge-Aronis, R. The response regulator RssB controls stability of the σ(S) subunit of RNA polymerase in Escherichia coli. EMBO J. 15, 1333–1339 (1996).
Zhou, Y., Gottesman, S., Hoskins, J.R., Maurizi, M.R. & Wickner, S. The RssB response regulator directly targets s(S) for degradation by ClpXP. Genes Dev. 15, 627–637 (2001).
Bougdour, A., Wickner, S. & Gottesman, S. Modulating RssB activity: IraP, a novel regulator of s(S) stability in Escherichia coli. Genes Dev. 20, 884–897 (2006).
Bachmair, A., Finley, D. & Varshavsky, A. In vivo half-life of a protein is a function of its amino-terminal residue. Science 234, 179–186 (1986).
Tobias, J.W., Shrader, T.E., Rocap, G. & Varshavsky, A. The N-end rule in bacteria. Science 254, 1374–1377 (1991).
Erbse, A. et al. ClpS is an essential component of the N-end rule pathway in Escherichia coli. Nature 439, 753–756 (2006).
Dougan, D.A., Reid, B.G., Horwich, A.L. & Bukau, B. ClpS, a substrate modulator of the ClpAP machine. Mol. Cell 9, 673–683 (2002).
Mogk, A., Schmidt, R. & Bukau, B. The N-end rule pathway for regulated proteolysis: prokaryotic and eukaryotic strategies. Trends Cell Biol. 17, 165–172 (2007).
Wang, K.H., Roman-Hernandez, G., Grant, R.A., Sauer, R.T. & Baker, T.A. The molecular basis of N-end rule recognition. Mol. Cell 32, 406–414 (2008).
Lupas, A.N. & Koretke, K.K. Bioinformatic analysis of ClpS, a protein module involved in prokaryotic and eukaryotic protein degradation. J. Struct. Biol. 141, 77–83 (2003).
Schuenemann, V.J. et al. Structural basis of N-end rule substrate recognition in Escherichia coli by the ClpAP adaptor protein ClpS. EMBO Rep. 10, 508–514 (2009).
Koodathingal, P. et al. ATP-dependent proteases differ substantially in their ability to unfold globular proteins. J. Biol. Chem. 284, 18674–18684 (2009).
Bence, N.F., Sampat, R.M. & Kopito, R.R. Impairment of the ubiquitin-proteasome system by protein aggregation. Science 292, 1552–1555 (2001).
Kaganovich, D., Kopito, R. & Frydman, J. Misfolded proteins partition between two distinct quality control compartments. Nature 454, 1088–1095 (2008).
Banaszynski, L.A. & Wandless, T.J. Conditional control of protein function. Chem. Biol. 13, 11–21 (2006).
Matsuzawa, S., Cuddy, M., Fukushima, T. & Reed, J.C. Method for targeting protein destruction by using a ubiquitin-independent, proteasome-mediated degradation pathway. Proc. Natl. Acad. Sci. USA 102, 14982–14987 (2005).
Spencer, D.M., Wandless, T.J., Schreiber, S.L. & Crabtree, G.R. Controlling signal transduction with synthetic ligands. Science 262, 1019–1024 (1993).
Liberles, S.D., Diver, S.T., Austin, D.J. & Schreiber, S.L. Inducible gene expression and protein translocation using nontoxic ligands identified by a mammalian three-hybrid screen. Proc. Natl. Acad. Sci. USA 94, 7825–7830 (1997).
Janse, D.M., Crosas, B., Finley, D. & Church, G.M. Localization to the proteasome is sufficient for degradation. J. Biol. Chem. 279, 21415–21420 (2004).
Stankunas, K. et al. Conditional protein alleles using knockin mice and a chemical inducer of dimerization. Mol. Cell 12, 1615–1624 (2003).
Banaszynski, L.A., Chen, L.-C., Maynard-Smith, L.A., Ooi, A.G.L. & Wandless, T.J.A. Rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell 126, 995–1004 (2006).
Pratt, M.R., Schwartz, E.C. & Muir, T.W. Small-molecule-mediated rescue of protein function by an inducible proteolytic shunt. Proc. Natl. Acad. Sci. USA 104, 11209–11214 (2007).
Gosink, M.M. & Vierstra, R.D. Redirecting the specificity of ubiquitination by modifying ubiquitin-conjugating enzymes. Proc. Natl. Acad. Sci. USA 92, 9117–9121 (1995).
Zhou, P., Bogacki, R., McReynolds, L. & Howley, P.M. Harnessing the ubiquitination machinery to target the degradation of specific cellular proteins. Mol. Cell 6, 751–756 (2000).
Su, Y., Ishikawa, S., Kojima, M. & Liu, B. Eradication of pathogenic β-catenin by Skp1/Cullin/F box ubiquitination machinery. Proc. Natl. Acad. Sci. USA 100, 12729–12734 (2003).
Sakamoto, K.M. et al. Protacs: chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc. Natl. Acad. Sci. USA 98, 8554–8559 (2001).
Schneekloth, J.S. et al. Chemical genetic control of protein levels: selective in vivo targeted degradation. J. Am. Chem. Soc. 126, 3748–3754 (2004).
Park, E.C., Finley, D. & Szostak, J.W. A strategy for the generation of conditional mutations by protein destabilization. Proc. Natl. Acad. Sci. USA 89, 1249–1252 (1992).
Dohmen, R.J., Wu, P. & Varshavsky, A. Heat-inducible degron: a method for constructing temperature-sensitive mutants. Science 263, 1273–1276 (1994).
Acknowledgements
We thank members of the Matouschek lab for advice and comments. The work was supported by grant R01GM64003 from the US National Institutes of Health, by grant MCB 426913 from the US National Science Foundation and by the Robert H. Lurie Comprehensive Cancer Center at Northwestern University. E.K.S. was supported by the Oncogenesis and Developmental Biology training grant T32 CA080621 from the US National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Schrader, E., Harstad, K. & Matouschek, A. Targeting proteins for degradation. Nat Chem Biol 5, 815–822 (2009). https://doi.org/10.1038/nchembio.250
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.250
This article is cited by
-
Degron masking outlines degronons, co-degrading functional modules in the proteome
Communications Biology (2022)
-
Linking post-translational modifications and protein turnover by site-resolved protein turnover profiling
Nature Communications (2022)
-
Predisposition to atrioventricular septal defects may be caused by SOX7 variants that impair interaction with GATA4
Molecular Genetics and Genomics (2022)
-
Improved pyrrolysine biosynthesis through phage assisted non-continuous directed evolution of the complete pathway
Nature Communications (2021)
-
ACSL4 promotes hepatocellular carcinoma progression via c-Myc stability mediated by ERK/FBW7/c-Myc axis
Oncogenesis (2020)