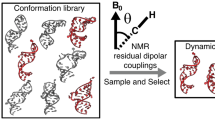
Abstract
Molecular recognition is central to all biological processes. For the past 50 years, Koshland's 'induced fit' hypothesis has been the textbook explanation for molecular recognition events. However, recent experimental evidence supports an alternative mechanism. 'Conformational selection' postulates that all protein conformations pre-exist, and the ligand selects the most favored conformation. Following binding the ensemble undergoes a population shift, redistributing the conformational states. Both conformational selection and induced fit appear to play roles. Following binding by a primary conformational selection event, optimization of side chain and backbone interactions is likely to proceed by an induced fit mechanism. Conformational selection has been observed for protein-ligand, protein-protein, protein-DNA, protein-RNA and RNA-ligand interactions. These data support a new molecular recognition paradigm for processes as diverse as signaling, catalysis, gene regulation and protein aggregation in disease, which has the potential to significantly impact our views and strategies in drug design, biomolecular engineering and molecular evolution.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
28 October 2009
In the version of this article initially published, in Figure 3 the term "free energy" appears with the horizontal axes rather than the vertical axes of the energy diagrams. The error has been corrected in the HTML and PDF versions of the article.
References
Fischer, E. Einfluss der configuration auf die wirkung der enzyme. Ber. Dtsch. Chem. Ges. 27, 2984–2993 (1894).
Koshland, D.E. Application of a theory of enzyme specificity to protein synthesis. Proc. Natl. Acad. Sci. USA 44, 98–104 (1958).
Frauenfelder, H., Sligar, S.G. & Wolynes, P.G. The energy landscapes and motions of proteins. Science 254, 1598–1603 (1991).
Ma, B., Kumar, S., Tsai, C.J. & Nussinov, R. Folding funnels and binding mechanisms. Protein Eng. 12, 713–720 (1999).
Tsai, C.J., Kumar, S., Ma, B. & Nussinov, R. Folding funnels, binding funnels, and protein function. Protein Sci. 8, 1181–1190 (1999).
Tsai, C.J., Ma, B. & Nussinov, R. Folding and binding cascades: shifts in energy landscapes. Proc. Natl. Acad. Sci. USA 96, 9970–9972 (1999).
Foote, J. & Milstein, C. Conformational isomerism and the diversity of antibodies. Proc. Natl. Acad. Sci. USA 91, 10370–10374 (1994).
Bosshard, H.R. Molecular recognition by induced fit: how fit is the concept? News Physiol. Sci. 16, 171–173 (2001).
Berger, C. et al. Antigen recognition by conformational selection. FEBS Lett. 450, 149–153 (1999).
Leder, L. et al. Spectroscopic, calorimetric, and kinetic demonstration of conformational adaptation in peptide-antibody recognition. Biochemistry 34, 16509–16518 (1995).
Kumar, S., Ma, B., Tsai, C.J., Sinha, N. & Nussinov, R. Folding and binding cascades: dynamic landscapes and population shifts. Protein Sci. 9, 10–19 (2000).
Miller, D.W. & Dill, K.A. Ligand binding to proteins: the binding landscape model. Protein Sci. 6, 2166–2179 (1997).
Dill, K.A. & Chan, H.S. From Levinthal to pathways to funnels. Nat. Struct. Biol. 4, 10–19 (1997).
Ma, B., Shatsky, M., Wolfson, H.J. & Nussinov, R. Multiple diverse ligands binding at a single protein site: a matter of pre-existing populations. Protein Sci. 11, 184–197 (2002).
Greenleaf, W.J., Woodside, M.T. & Block, S.M. High-resolution, single-molecule measurements of biomolecular motion. Annu. Rev. Biophys. Biomol. Struct. 36, 171–190 (2007).
Parak, F.G. Proteins in action: the physics of structural fluctuations and conformational changes. Curr. Opin. Struct. Biol. 13, 552–557 (2003).
Hinterdorfer, P. & Dufrene, Y.F. Detection and localization of single molecular recognition events using atomic force microscopy. Nat. Methods 3, 347–355 (2006).
Busenlehner, L.S. & Armstrong, R.N. Insights into enzyme structure and dynamics elucidated by amide H/D exchange mass spectrometry. Arch. Biochem. Biophys. 433, 34–46 (2005).
Palmer, A.G. III. Nmr probes of molecular dynamics: overview and comparison with other techniques. Annu. Rev. Biophys. Biomol. Struct. 30, 129–155 (2001).
Tobi, D. & Bahar, I. Structural changes involved in protein binding correlate with intrinsic motions of proteins in the unbound state. Proc. Natl. Acad. Sci. USA 102, 18908–18913 (2005).
Keskin, O. Binding induced conformational changes of proteins correlate with their intrinsic fluctuations: a case study of antibodies. BMC Struct. Biol. 7, 31 (2007).
Grunberg, R., Leckner, J. & Nilges, M. Complementarity of structure ensembles in protein-protein binding. Structure 12, 2125–2136 (2004).
Austin, R.H., Beeson, K.W., Eisenstein, L., Frauenfelder, H. & Gunsalus, I.C. Dynamics of ligand binding to myoglobin. Biochemistry 14, 5355–5373 (1975).
Schotte, F. et al. Watching a protein as it functions with 150-ps time-resolved x-ray crystallography. Science 300, 1944–1947 (2003).
Vos, M.H. Ultrafast dynamics of ligands within heme proteins. Biochim. Biophys. Acta 1777, 15–31 (2008).
Lee, A.Y., Gulnik, S.V. & Erickson, J.W. Conformational switching in an aspartic proteinase. Nat. Struct. Biol. 5, 866–871 (1998).
Henzler-Wildman, K.A. et al. Intrinsic motions along an enzymatic reaction trajectory. Nature 450, 838–844 (2007). This study provides experimental support from X-ray crystallography, NMR and single-molecule fluorescence that adenylate kinase fluctuates between open and closed states in the absence of ligand.
Muller, Y.A., Kelley, R.F. & de Vos, A.M. Hinge bending within the cytokine receptor superfamily revealed by the 2.4 A crystal structure of the extracellular domain of rabbit tissue factor. Protein Sci. 7, 1106–1115 (1998).
James, L.C., Roversi, P. & Tawfik, D.S. Antibody multispecificity mediated by conformational diversity. Science 299, 1362–1367 (2003).
Hanes, J., Jermutus, L., Weber-Bornhauser, S., Bosshard, H.R. & Pluckthun, A. Ribosome display efficiently selects and evolves high-affinity antibodies in vitro from immune libraries. Proc. Natl. Acad. Sci. USA 95, 14130–14135 (1998).
Stella, L. et al. Flexibility of helix 2 in the human glutathione transferase P1–1. time-resolved fluorescence spectroscopy. J. Biol. Chem. 273, 23267–23273 (1998).
Xu, J. & Root, D.D. Conformational selection during weak binding at the actin and myosin interface. Biophys. J. 79, 1498–1510 (2000).
Cao, Y., Musah, R.A., Wilcox, S.K., Goodin, D.B. & McRee, D.E. Protein conformer selection by ligand binding observed with crystallography. Protein Sci. 7, 72–78 (1998).
Mittermaier, A. & Kay, L.E. New tools provide new insights in NMR studies of protein dynamics. Science 312, 224–228 (2006).
Cavalli, A., Salvatella, X., Dobson, C.M. & Vendruscolo, M. Protein structure determination from NMR chemical shifts. Proc. Natl. Acad. Sci. USA 104, 9615–9620 (2007).
Vallurupalli, P., Hansen, D.F., Stollar, E., Meirovitch, E. & Kay, L.E. Measurement of bond vector orientations in invisible excited states of proteins. Proc. Natl. Acad. Sci. USA 104, 18473–18477 (2007).
Igumenova, T.I., Brath, U., Akke, M. & Palmer, A.G. III. Characterization of chemical exchange using residual dipolar coupling. J. Am. Chem. Soc. 129, 13396–13397 (2007).
Hansen, D.F., Vallurupalli, P. & Kay, L.E. Using relaxation dispersion NMR spectroscopy to determine structures of excited, invisible protein states. J. Biomol. NMR 41, 113–120 (2008).
Beach, H., Cole, R., Gill, M.L. & Loria, J.P. Conservation of mus-ms enzyme motions in the apo- and substrate-mimicked state. J. Am. Chem. Soc. 127, 9167–9176 (2005).
Wolf-Watz, M. et al. Linkage between dynamics and catalysis in a thermophilic-mesophilic enzyme pair. Nat. Struct. Mol. Biol. 11, 945–949 (2004).
Boehr, D.D., McElheny, D., Dyson, H.J. & Wright, P.E. The dynamic energy landscape of dihydrofolate reductase catalysis. Science 313, 1638–1642 (2006). This study suggests that every functional intermediate of dihydrofolate reductase fluctuates into a higher energy conformation that is structurally similar to the next and/or previous complex in the catalytic cycle.
McElheny, D., Schnell, J.R., Lansing, J.C., Dyson, H.J. & Wright, P.E. Defining the role of active-site loop fluctuations in dihydrofolate reductase catalysis. Proc. Natl. Acad. Sci. USA 102, 5032–5037 (2005).
Hanson, J.A. et al. Illuminating the mechanistic roles of enzyme conformational dynamics. Proc. Natl. Acad. Sci. USA 104, 18055–18060 (2007).
Eisenmesser, E.Z. et al. Intrinsic dynamics of an enzyme underlies catalysis. Nature 438, 117–121 (2005).
Antikainen, N.M., Smiley, R.D., Benkovic, S.J. & Hammes, G.G. Conformation coupled enzyme catalysis: single-molecule and transient kinetics investigation of dihydrofolate reductase. Biochemistry 44, 16835–16843 (2005).
Boehr, D.D., Dyson, H.J. & Wright, P.E. Conformational relaxation following hydride transfer plays a limiting role in dihydrofolate reductase catalysis. Biochemistry 47, 9227–9233 (2008).
Kitahara, R. et al. High pressure NMR reveals active-site hinge motion of folate-bound Escherichia coli dihydrofolate reductase. Biochemistry 39, 12789–12795 (2000).
Mauldin, R.V., Carroll, M.J. & Lee, A.L. Dynamic dysfunction in dihydrofolate reductase results from antifolate drug binding: modulation of dynamics within a structural state. Structure 17, 386–394 (2009).
Tang, C., Schwieters, C.D. & Clore, G.M. Open-to-closed transition in apo maltose-binding protein observed by paramagnetic NMR. Nature 449, 1078–1082 (2007).
Lu, Z.L., Coetsee, M., White, C.D. & Millar, R.P. Structural determinants for ligand-receptor conformational selection in a peptide G protein-coupled receptor. J. Biol. Chem. 282, 17921–17929 (2007).
Fenwick, R. et al. Solution structure and dynamics of the small GTPase RalB in its active conformation: significance for effector protein binding. Biochemistry 48, 2192–2206 (2009).
Saitoh, T. et al. Tom20 recognizes mitochondrial presequences through dynamic equilibrium among multiple bound states. EMBO J. 26, 4777–4787 (2007).
Brath, U. & Akke, M. Differential responses of the backbone and side chain conformational dynamics in FKBP12 upon binding the transition state analog FK506: implications for transition state stabilization and target protein recognition. J. Mol. Biol. 387, 233–244 (2009).
Keramisanou, D. et al. Disorder-order folding transitions underlie catalysis in the helicase motor of SecA. Nat. Struct. Mol. Biol. 13, 594–602 (2006).
Gsponer, J. et al. A coupled equilibrium shift mechanism in calmodulin-mediated signal transduction. Structure 16, 736–746 (2008).
Nevo, R. et al. A molecular switch between alternative conformational states in the complex of Ran and importin beta1. Nat. Struct. Biol. 10, 553–557 (2003).
Junker, J.P., Ziegler, F. & Rief, M. Ligand-dependent equilibrium fluctuations of single calmodulin molecules. Science 323, 633–637 (2009).
Koglin, A. et al. Conformational switches modulate protein interactions in peptide antibiotic synthetases. Science 312, 273–276 (2006).
Koglin, A. et al. Structural basis for the selectivity of the external thioesterase of the surfactin synthetase. Nature 454, 907–911 (2008).
Lange, O.F. et al. Recognition dynamics up to microseconds revealed from an RDC-derived ubiquitin ensemble in solution. Science 320, 1471–1475 (2008). This report presents an NMR-derived conformational ensemble of ubiquitin that encompasses all the crystallographically determined conformations of ubiquitin.
Kalodimos, C. et al. Structure and flexibility adaptation in nonspecific and specific protein-DNA complexes. Science 305, 386–389 (2004). This study compares the structure and dynamics of the lac repressor headpiece when bound to cognate versus noncognate DNA, and demonstrates that the underlying energy landscapes are distinct.
Zhang, Q., Stelzer, A.C., Fisher, C.K. & Al-Hashimi, H.M. Visualizing spatially correlated dynamics that directs RNA conformational transitions. Nature 450, 1263–1267 (2007). This study demonstrates that TAR RNA fluctuates into multiple “bound” conformations in the absence of ligands.
Al-Hashimi, H.M. & Walter, N.G. RNA dynamics: it is about time. Curr. Opin. Struct. Biol. 18, 321–329 (2008).
Mittag, T. & Forman-Kay, J.D. Atomic-level characterization of disordered protein ensembles. Curr. Opin. Struct. Biol. 17, 3–14 (2007).
Lakomek, N.A. et al. Residual dipolar couplings as a tool to study molecular recognition of ubiquitin. Biochem. Soc. Trans. 36, 1433–1437 (2008).
Clore, G.M., Tang, C. & Iwahara, J. Elucidating transient macromolecular interactions using paramagnetic relaxation enhancement. Curr. Opin. Struct. Biol. 17, 603–616 (2007).
Lindorff-Larsen, K., Best, R.B., Depristo, M.A., Dobson, C.M. & Vendruscolo, M. Simultaneous determination of protein structure and dynamics. Nature 433, 128–132 (2005).
Lipari, G. & Szabo, A. Nuclear magnetic resonance relaxation in nucleic acid fragments: models for internal motion. Biochemistry 20, 6250–6256 (1981).
Lakomek, N.A., Carlomagno, T., Becker, S., Griesinger, C. & Meiler, J. A thorough dynamic interpretation of residual dipolar couplings in ubiquitin. J. Biomol. NMR 34, 101–115 (2006).
Esler, W.P. et al. Alzheimer's disease amyloid propagation by a template-dependent dock-lock mechanism. Biochemistry 39, 6288–6295 (2000).
Tessier, P.M. & Lindquist, S. Prion recognition elements govern nucleation, strain specificity and species barriers. Nature 447, 556–561 (2007).
Kalodimos, C., Boelens, R. & Kaptein, R. Toward an integrated model of protein−DNA recognition as inferred from NMR studies on the lac repressor system. Chem. Rev. 104, 3567–3586 (2004).
von Hippel, P.H. & Berg, O.G. Facilitated target location in biological systems. J. Biol. Chem. 264, 675–678 (1989).
Gorman, J. & Greene, E.C. Visualizing one-dimensional diffusion of proteins along DNA. Nat. Struct. Mol. Biol. 15, 768–774 (2008).
Zhang, Q., Stelzer, A., Fisher, C. & Al-Hashimi, H. Visualizing spatially correlated dynamics that directs RNA conformational transitions. Nature 450, 1263–1267 (2007).
Monod, J., Wyman, J. & Changeux, J.P. On the nature of allosteric transitions: A plausible model. J. Mol. Biol. 12, 88–118 (1965).
Kantrowitz, E.R. & Lipscomb, W.N. Escherichia coli aspartate transcarbamoylase: the molecular basis for a concerted allosteric transition. Trends Biochem. Sci. 15, 53–59 (1990).
Velyvis, A., Yang, Y.R., Schachman, H.K. & Kay, L.E. A solution NMR study showing that active site ligands and nucleotides directly perturb the allosteric equilibrium in aspartate transcarbamoylase. Proc. Natl. Acad. Sci. USA 104, 8815–8820 (2007).
Velyvis, A., Schachman, H.K. & Kay, L.E. Application of methyl-TROSY NMR to test allosteric models describing effects of nucleotide binding to aspartate transcarbamoylase. J. Mol. Biol. 387, 540–547 (2009).
Gunasekaran, K., Ma, B. & Nussinov, R. Is allostery an intrinsic property of all dynamic proteins? Proteins 57, 433–443 (2004).
Volkman, B.F., Lipson, D., Wemmer, D.E. & Kern, D. Two-state allosteric behavior in a single-domain signaling protein. Science 291, 2429–2433 (2001).
Yao, X., Rosen, M.K. & Gardner, K.H. Estimation of the available free energy in a LOV2-J alpha photoswitch. Nat. Chem. Biol. 4, 491–497 (2008).
Li, P., Martins, I.R., Amarasinghe, G.K. & Rosen, M.K. Internal dynamics control activation and activity of the autoinhibited Vav DH domain. Nat. Struct. Mol. Biol. 15, 613–618 (2008). This study presents a linear correlation between biological activity and the population of the Vav DH higher energy conformation. This provides both structural and functional evidence for the involvement of a higher energy conformation in biological function.
Boehr, D.D., Dyson, H.J. & Wright, P.E. An NMR perspective on enzyme dynamics. Chem. Rev. 106, 3055–3079 (2006).
Lee, G.M. & Craik, C.S. Trapping moving targets with small molecules. Science 324, 213–215 (2009).
Bursavich, M.G. & Rich, D.H. Designing non-peptide peptidomimetics in the 21st century: inhibitors targeting conformational ensembles. J. Med. Chem. 45, 541–558 (2002).
Totrov, M. & Abagyan, R. Flexible ligand docking to multiple receptor conformations: a practical alternative. Curr. Opin. Struct. Biol. 18, 178–184 (2008).
Andrusier, N., Mashiach, E., Nussinov, R. & Wolfson, H.J. Principles of flexible protein-protein docking. Proteins 73, 271–289 (2008).
Chaudhury, S. & Gray, J.J. Conformer selection and induced fit in flexible backbone protein-protein docking using computational and NMR ensembles. J. Mol. Biol. 381, 1068–1087 (2008).
Lindsley, C.W. & Emmitte, K.A. Recent progress in the discovery and development of negative allosteric modulators of mGluR5. Curr. Opin. Drug Discov. Devel. 12, 446–457 (2009).
Frederick, K.K., Marlow, M.S., Valentine, K.G. & Wand, A.J. Conformational entropy in molecular recognition by proteins. Nature 448, 325–329 (2007).
Rothlisberger, D. et al. Kemp elimination catalysts by computational enzyme design. Nature 453, 190–195 (2008).
Jiang, L. et al. De novo computational design of retro-aldol enzymes. Science 319, 1387–1391 (2008).
Hilvert, D. Critical analysis of antibody catalysis. Annu. Rev. Biochem. 69, 751–793 (2000).
Pauling, L. & Delbruck, M. The nature of the intermolecular forces operative in biological processes. Science 92, 77–79 (1940).
James, L.C. & Tawfik, D.S. Conformational diversity and protein evolution—a 60-year-old hypothesis revisited. Trends Biochem. Sci. 28, 361–368 (2003).
Tokuriki, N. & Tawfik, D.S. Protein dynamism and evolvability. Science 324, 203–207 (2009).
Wedemayer, G.J., Patten, P.A., Wang, L.H., Schultz, P.G. & Stevens, R.C. Structural insights into the evolution of an antibody combining site. Science 276, 1665–1669 (1997).
Weikl, T. & Von Deuster, C. Selected-fit versus induced-fit protein binding: kinetic differences and mutational analysis. Proteins 75, 104–110 (2009).
Acknowledgements
This work was supported by the US National Institutes of Health (NIH grants GM75995 and CA96865 to P.E.W.) and by the Skaggs Institute for Chemical Biology. This project has been funded in whole or in part with federal funds from the National Cancer Institute, NIH, under contract number N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government. This research was supported (in part) by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Boehr, D., Nussinov, R. & Wright, P. The role of dynamic conformational ensembles in biomolecular recognition. Nat Chem Biol 5, 789–796 (2009). https://doi.org/10.1038/nchembio.232
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.232
This article is cited by
-
Unlocking the potential of RNAi as a therapeutic strategy against infectious viruses: an in-silico study
Chemical Papers (2024)
-
Chemiresistive sensing with functionalized carbon nanotubes
Nature Reviews Methods Primers (2023)
-
Preferential molecular recognition of heterochiral guests within a cyclophane receptor
Nature Communications (2023)
-
Unexpected dynamics in femtomolar complexes of binding proteins with peptides
Nature Communications (2023)
-
Discovery of a selective and biologically active low-molecular weight antagonist of human interleukin-1β
Nature Communications (2023)