Abstract
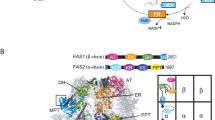
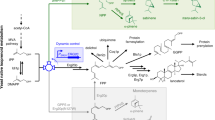
Fungal type I fatty acid synthases (FASs) are mega-enzymes with two separated, identical compartments, in which the acyl carrier protein (ACP) domains shuttle substrates to catalytically active sites embedded in the chamber wall. We devised synthetic FASs by integrating heterologous enzymes into the reaction chambers and demonstrated their capability to convert acyl-ACP or acyl-CoA from canonical fatty acid biosynthesis to short/medium-chain fatty acids and methyl ketones.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Accession codes
References
Jenni, S. et al. Science 316, 254–261 (2007).
Lomakin, I.B., Xiong, Y. & Steitz, T.A. Cell 129, 319–332 (2007).
Zhu, Z. et al. Nat. Commun. 3, 1112 (2012).
Fischer, M. et al. Protein Sci. 24, 987–995 (2015).
Zhu, Z., Zhang, S., Lin, X., Liu, W. & Zhao, Z.K. Chin. J. Biotechnol. 30, 1414–1423 (2014).
Zheng, Y. et al. Biotechnol. Biofuels 5, 76 (2012).
Gajewski, J. et al. Nat. Chem. Biol. 13 10.1038/nchembio.2314 (2017).
Leber, C., Choi, J.W., Polson, B. & Da Silva, N.A. Biotechnol. Bioeng. 113, 895–900 (2016).
Yu, G. et al. Plant Physiol. 154, 67–77 (2010).
Schweizer, E. & Hofmann, J. Microbiol. Mol. Biol. Rev. 68, 501–517 (2004).
Aprahamian, S.A., Arslanian, M.J. & Wakil, S.J. Comp. Biochem. Physiol. B. 71, 577–582 (1982).
Johansson, P. et al. Proc. Natl. Acad. Sci. USA 105, 12803–12808 (2008).
Maier, T., Leibundgut, M., Boehringer, D. & Ban, N. Q. Rev. Biophys. 43, 373–422 (2010).
Leber, C. & Da Silva, N.A. Biotechnol. Bioeng. 111, 347–358 (2014).
Zhou, Y.J. et al. Nat. Commun. 7, 11709 (2016).
Sambrook, J. & Russell, D.W. Molecular Cloning: a Laboratory Manual 3rd edn. (Cold Spring Harbor Laboratory Press, 2001).
van den Ent, F. & Löwe, J. J. Biochem. Biophys. Methods 67, 67–74 (2006).
Shao, Z., Zhao, H. & Zhao, H. Nucleic Acids Res. 37, e16 (2009).
Zhou, Y.J. et al. J. Am. Chem. Soc. 134, 3234–3241 (2012).
Wenz, P., Schwank, S., Hoja, U. & Schüller, H.-J. Nucleic Acids Res. 29, 4625–4632 (2001).
Wang, J., Zhang, S., Tan, H. & Zhao, Z.K. J. Microbiol. Methods 71, 225–230 (2007).
Schägger, H. Nat. Protoc. 1, 16–22 (2006).
Reid, R.J.D., Sunjevaric, I., Keddache, M. & Rothstein, R. Yeast 19, 319–328 (2002).
Gueldener, U., Heinisch, J., Koehler, G.J., Voss, D. & Hegemann, J.H. Nucleic Acids Res. 30, e23 (2002).
Jensen, N.B. et al. FEMS Yeast Res. 14, 238–248 (2014).
Mitchell, J., Smith, D.M. & Bryant, W.M.D. J. Am. Chem. Soc. 62, 4–6 (1940).
Khoomrung, S., Chumnanpuen, P., Jansa-ard, S., Nookaew, I. & Nielsen, J. Appl. Microbiol. Biotechnol. 94, 1637–1646 (2012).
Acknowledgements
This work was funded by grants from Total New Energies, the Novo Nordisk Foundation, and the Knut and Alice Wallenberg Foundation to J.N. and from the National Natural Science Foundation of China to Z.K.Z. (No. 21325627). We thank H.-J. Schüller (Ernst-Moritz-Arndt-Universität Greifswald, Germany) for sharing the PWY12 strain; J.L. Collier (Stony Brook University, USA) for providing genomic DNA of A. kerguelense; C. Song and J. Li (Dalian Institute of Chemical Physics), respectively, for the MALDI–TOF Mass Spectrometry analysis and ultracentrifugation; and S. Khoomrung and the Chalmers Mass Spectrometry Infrastructure (J. Karlsson and J. Kindbom) for assistance with GC–MS analysis. We also appreciate the help from and/or discussion with S. Zhang, W. Liu, X. Lin, V. Siewers, Y. Chen, M. Fischer, Z. Dai, N.A. Buijs, P. Teixeira and O. Vidalin.
Author information
Authors and Affiliations
Contributions
Z.Z., Z.K.Z. and J.N. conceived this study. Z.Z. designed and performed most of the experiments and analyzed the data. Z.Z. and Z.K.Z. performed biochemical study of Rhodosporidium fatty acid synthase (FAS) at Dalian Institute of Chemical Physics. Y.J.Z. participated in the plasmid construction and product quantification. A.K. assisted with data analysis and interpretation. M.G. developed the ketoacyl synthase (KS) mutation. Z.Z. and J.N. wrote the manuscript. All authors revised and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
Z.Z., A.K. and J.N. are listed as co-inventors on a patent application related to fatty acid production. J.N. and A.K. are shareholders in Biopetrolia AB.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Tables 1–7 and Supplementary Figures 1–9. (PDF 7299 kb)
Rights and permissions
About this article
Cite this article
Zhu, Z., Zhou, Y., Krivoruchko, A. et al. Expanding the product portfolio of fungal type I fatty acid synthases. Nat Chem Biol 13, 360–362 (2017). https://doi.org/10.1038/nchembio.2301
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2301
This article is cited by
-
Increased CO2 fixation enables high carbon-yield production of 3-hydroxypropionic acid in yeast
Nature Communications (2024)
-
An optimized reverse β-oxidation pathway to produce selected medium-chain fatty acids in Saccharomyces cerevisiae
Biotechnology for Biofuels and Bioproducts (2023)
-
Synthetic biology for sustainable food ingredients production: recent trends
Systems Microbiology and Biomanufacturing (2023)
-
Engineering Saccharomyces cerevisiae for production of the capsaicinoid nonivamide
Microbial Cell Factories (2022)
-
Mucor circinelloides: a model organism for oleaginous fungi and its potential applications in bioactive lipid production
Microbial Cell Factories (2022)