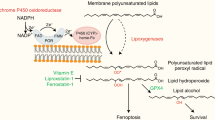
Abstract
Enigmatic lipid peroxidation products have been claimed as the proximate executioners of ferroptosis—a specialized death program triggered by insufficiency of glutathione peroxidase 4 (GPX4). Using quantitative redox lipidomics, reverse genetics, bioinformatics and systems biology, we discovered that ferroptosis involves a highly organized oxygenation center, wherein oxidation in endoplasmic-reticulum-associated compartments occurs on only one class of phospholipids (phosphatidylethanolamines (PEs)) and is specific toward two fatty acyls—arachidonoyl (AA) and adrenoyl (AdA). Suppression of AA or AdA esterification into PE by genetic or pharmacological inhibition of acyl-CoA synthase 4 (ACSL4) acts as a specific antiferroptotic rescue pathway. Lipoxygenase (LOX) generates doubly and triply-oxygenated (15-hydroperoxy)-diacylated PE species, which act as death signals, and tocopherols and tocotrienols (vitamin E) suppress LOX and protect against ferroptosis, suggesting a homeostatic physiological role for vitamin E. This oxidative PE death pathway may also represent a target for drug discovery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Allocati, N., Masulli, M., Di Ilio, C. & De Laurenzi, V. Die for the community: an overview of programmed cell death in bacteria. Cell Death Dis. 6, e1609 (2015).
Byrne, J.M. et al. Redox cycling of Fe(II) and Fe(III) in magnetite by Fe-metabolizing bacteria. Science 347, 1473–1476 (2015).
Dixon, S.J. & Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 10, 9–17 (2014).
Dixon, S.J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Yang, W.S. & Stockwell, B.R. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 26, 165–176 (2016).
Yang, W.S. & Stockwell, B.R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 15, 234–245 (2008).
Imai, H. & Nakagawa, Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic. Biol. Med. 34, 145–169 (2003).
Yamanaka, K. et al. A novel fluorescent probe with high sensitivity and selective detection of lipid hydroperoxides in cells. RSC Advances 2, 7894–7900 (2012).
Drummen, G.P., van Liebergen, L.C., Op den Kamp, J.A. & Post, J.A. C11-BODIPY581/591, an oxidation-sensitive fluorescent lipid peroxidation probe: (micro)spectroscopic characterization and validation of methodology. Free Radic. Biol. Med. 33, 473–490 (2002).
Li, B. & Pratt, D.A. Methods for determining the efficacy of radical-trapping antioxidants. Free Radic. Biol. Med. 82, 187–202 (2015).
Küch, E.M. et al. Differentially localized acyl-CoA synthetase 4 isoenzymes mediate the metabolic channeling of fatty acids towards phosphatidylinositol. Biochim. Biophys. Acta 1841, 227–239 (2014).
Golej, D.L. et al. Long-chain acyl-CoA synthetase 4 modulates prostaglandin E release from human arterial smooth muscle cells. J. Lipid Res. 52, 782–793 (2011).
Doll, S. et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. http://dx.doi.org/10.1038/nchembio.2239 (2016).
McIntyre, T.M., Prescott, S.M. & Stafforini, D.M. The emerging roles of PAF acetylhydrolase. J. Lipid Res. 50 (Suppl.): S255–S259 (2009).
Soh, N. et al. Swallow-tailed perylene derivative: a new tool for fluorescent imaging of lipid hydroperoxides. Org. Biomol. Chem. 5, 3762–3768 (2007).
Dixon, S.J. et al. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem. Biol. 10, 1604–1609 (2015).
Seiler, A. et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 8, 237–248 (2008).
Friedmann Angeli, J.P. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16, 1180–1191 (2014).
Askari, B. et al. Rosiglitazone inhibits acyl-CoA synthetase activity and fatty acid partitioning to diacylglycerol and triacylglycerol via a peroxisome proliferator-activated receptor-γ-independent mechanism in human arterial smooth muscle cells and macrophages. Diabetes 56, 1143–1152 (2007).
O'Donnell, V.B. & Murphy, R.C. New families of bioactive oxidized phospholipids generated by immune cells: identification and signaling actions. Blood 120, 1985–1992 (2012).
Xiao, Y. & Guengerich, F.P. Metabolomic analysis and identification of a role for the orphan human cytochrome P450 2W1 in selective oxidation of lysophospholipids. J. Lipid Res. 53, 1610–1617 (2012).
Khanna, S. et al. Molecular basis of vitamin E action: tocotrienol modulates 12-lipoxygenase, a key mediator of glutamate-induced neurodegeneration. J. Biol. Chem. 278, 43508–43515 (2003).
Arai, H., Nagao, A., Terao, J., Suzuki, T. & Takama, K. Effect of D-α-tocopherol analogues on lipoxygenase-dependent peroxidation of phospholipid–bile salt micelles. Lipids 30, 135–140 (1995).
Dennis, E.A. Diversity of group types, regulation, and function of phospholipase A2. J. Biol. Chem. 269, 13057–13060 (1994).
van den Brink-van der Laan, E., Killian, J.A. & de Kruijff, B. Nonbilayer lipids affect peripheral and integral membrane proteins via changes in the lateral pressure profile. Biochim. Biophys. Acta 1666, 275–288 (2004).
Lee, A.G. How lipids affect the activities of integral membrane proteins. Biochim. Biophys. Acta 1666, 62–87 (2004).
Toppo, S., Flohé, L., Ursini, F., Vanin, S. & Maiorino, M. Catalytic mechanisms and specificities of glutathione peroxidases: variations of a basic scheme. Biochim. Biophys. Acta 1790, 1486–1500 (2009).
Uderhardt, S. et al. 12/15-lipoxygenase orchestrates the clearance of apoptotic cells and maintains immunologic tolerance. Immunity 36, 834–846 (2012).
Orrenius, S. & Zhivotovsky, B. Cardiolipin oxidation sets cytochrome c free. Nat. Chem. Biol. 1, 188–189 (2005).
Kagan, V.E. et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 1, 223–232 (2005).
Massey, K.A. & Nicolaou, A. Lipidomics of polyunsaturated-fatty-acid-derived oxygenated metabolites. Biochem. Soc. Trans. 39, 1240–1246 (2011).
Kuhn, H., Banthiya, S. & van Leyen, K. Mammalian lipoxygenases and their biological relevance. Biochim. Biophys. Acta 1851, 308–330 (2015).
Schroeder, F. Regulation of aminophospholipid asymmetry in murine fibroblast plasma membranes by choline and ethanolamine analogues. Biochim. Biophys. Acta 599, 254–270 (1980).
Sessions, A. & Horwitz, A.F. Myoblast aminophospholipid asymmetry differs from that of fibroblasts. FEBS Lett. 134, 75–78 (1981).
Garreta, A. et al. Structure and interaction with phospholipids of a prokaryotic lipoxygenase from Pseudomonas aeruginosa. FASEB J. 27, 4811–4821 (2013).
Suardíaz, R. et al. Understanding the mechanism of the hydrogen abstraction from arachidonic acid catalyzed by the human enzyme 15-lipoxygenase-2. A quantum mechanics/molecular mechanics free energy simulation. J. Chem. Theory Comput. 12, 2079–2090 (2016).
Noguchi, N. et al. The specificity of lipoxygenase-catalyzed lipid peroxidation and the effects of radical-scavenging antioxidants. Biol. Chem. 383, 619–626 (2002).
Carlson, B.A. et al. Glutathione peroxidase 4 and vitamin E cooperatively prevent hepatocellular degeneration. Redox Biol. 9, 22–31 (2016).
Chen, L., Hambright, W.S., Na, R. & Ran, Q. Ablation of the ferroptosis inhibitor glutathione peroxidase 4 in neurons results in rapid motor neuron degeneration and paralysis. J. Biol. Chem. 290, 28097–28106 (2015).
Matsushita, M. et al. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J. Exp. Med. 212, 555–568 (2015).
Wortmann, M. et al. Combined deficiency in glutathione peroxidase 4 and vitamin E causes multiorgan thrombus formation and early death in mice. Circ. Res. 113, 408–417 (2013).
Sen, C.K., Khanna, S., Roy, S. & Packer, L. Molecular basis of vitamin E action. Tocotrienol potently inhibits glutamate-induced pp60c-Src kinase activation and death of HT4 neuronal cells. J. Biol. Chem. 275, 13049–13055 (2000).
Yang, W.S. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014).
Telmer, C.A. et al. Rapid, specific, no-wash, far-red fluorogen activation in subcellular compartments by targeted fluorogen activating proteins. ACS Chem. Biol. 10, 1239–1246 (2015).
Szent-Gyorgyi, C. et al. Fluorogen-activating single-chain antibodies for imaging cell surface proteins. Nat. Biotechnol. 26, 235–240 (2008).
Tardi, P.G., Mukherjee, J.J. & Choy, P.C. The quantitation of long-chain acyl-CoA in mammalian tissue. Lipids 27, 65–67 (1992).
Minkler, P.E., Kerner, J., Ingalls, S.T. & Hoppel, C.L. Novel isolation procedure for short-, medium-, and long-chain acyl–coenzyme A esters from tissue. Anal. Biochem. 376, 275–276 (2008).
Sun, D., Cree, M.G. & Wolfe, R.R. Quantification of the concentration and 13C tracer enrichment of long-chain fatty acyl–coenzyme A in muscle by liquid chromatography/mass spectrometry. Anal. Biochem. 349, 87–95 (2006).
Folch, J., Lees, M. & Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509 (1957).
Miller, T.M. et al. Rapid, simultaneous quantitation of mono and dioxygenated metabolites of arachidonic acid in human CSF and rat brain. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877, 3991–4000 (2009).
Tejero, J. et al. Peroxidase activation of cytoglobin by anionic phospholipids: mechanisms and consequences. Biochim. Biophys. Acta 1861, 391–401 (2016).
Kobe, M.J., Neau, D.B., Mitchell, C.E., Bartlett, S.G. & Newcomer, M.E. The structure of human 15-lipoxygenase-2 with a substrate mimic. J. Biol. Chem. 289, 8562–8569 (2014).
Marrink, S.J., Risselada, H.J., Yefimov, S., Tieleman, D.P. & de Vries, A.H. The MARTINI force field: coarse grained model for biomolecular simulations. J. Phys. Chem. B 111, 7812–7824 (2007).
Van Der Spoel, D. et al. GROMACS: fast, flexible, and free. J. Comput. Chem. 26, 1701–1718 (2005).
Yang, K. et al. Dynamic simulations on the arachidonic acid metabolic network. PLoS Comput. Biol. 3, e55 (2007).
Gupta, S., Maurya, M.R., Stephens, D.L., Dennis, E.A. & Subramaniam, S. An integrated model of eicosanoid metabolism and signaling based on lipidomics flux analysis. Biophys. J. 96, 4542–4551 (2009).
Hoops, S. et al. COPASI—a COmplex PAthway SImulator. Bioinformatics 22, 3067–3074 (2006).
Acknowledgements
We thank J. Ruzicka (Thermo Fisher Scientific) for help in obtaining MS3 spectra of PE oxidation products using tribrid Fusion Lumos. Supported by the US National Institutes of Health (P01HL114453 to R.K.M., U19AI068021 to J.G., NS076511 to V.E.K., NS061817 to H.B., P41GM103712 to I.B. and ES020693 to Y.Y.T.), the Human Frontier Science Program (HFSP-RGP0013/2014), and the Deutsche Forschungsgemeinschaft (CO 291/2-3 and CO 291/5-1) to M.C.
Author information
Authors and Affiliations
Contributions
V.E.K., M.C. and H.B. formulated the idea, designed the study and wrote the manuscript. G.M. and J.P.F.A. performed cell experiments. Y.Y.T. and F.Q. performed MS lipid analysis, interpreted data. C.S. and S.W. performed cell imaging experiments. T.A., V.A.T. and A.A.A. performed model systems experiments. D.M. and J.K.-S. performed computational modeling. B.L. and I.B. performed network analysis. S.D., H.H.D., J.J., V.B.R., A.A.K., B.P. and Q.Y. participated in cell or animal experiments. J.G., R.K.M. and B.R.S. participated in formulating the idea and writing the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Tables 1–5 and Supplementary Figures 1–18. (PDF 9201 kb)
Rights and permissions
About this article
Cite this article
Kagan, V., Mao, G., Qu, F. et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol 13, 81–90 (2017). https://doi.org/10.1038/nchembio.2238
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2238
This article is cited by
-
Harnessing ferroptosis for enhanced sarcoma treatment: mechanisms, progress and prospects
Experimental Hematology & Oncology (2024)
-
Ferroptosis in early brain injury after subarachnoid hemorrhage: review of literature
Chinese Neurosurgical Journal (2024)
-
Ferroptosis: a promising candidate for exosome-mediated regulation in different diseases
Cell Communication and Signaling (2024)
-
Role of ferroptosis in chronic kidney disease
Cell Communication and Signaling (2024)
-
The application of nanoparticles-based ferroptosis, pyroptosis and autophagy in cancer immunotherapy
Journal of Nanobiotechnology (2024)