Abstract
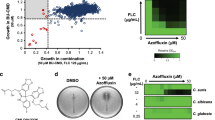
There is an urgent need for new strategies to treat invasive fungal infections, which are a leading cause of human mortality. Here, we establish two activities of the natural product beauvericin, which potentiates the activity of the most widely deployed class of antifungal against the leading human fungal pathogens, blocks the emergence of drug resistance, and renders antifungal-resistant pathogens responsive to treatment in mammalian infection models. Harnessing genome sequencing of beauvericin-resistant mutants, affinity purification of a biotinylated beauvericin analog, and biochemical and genetic assays reveals that beauvericin blocks multidrug efflux and inhibits the global regulator TORC1 kinase, thereby activating the protein kinase CK2 and inhibiting the molecular chaperone Hsp90. Substitutions in the multidrug transporter Pdr5 that enable beauvericin efflux impair antifungal efflux, thereby impeding resistance to the drug combination. Thus, dual targeting of multidrug efflux and TOR signaling provides a powerful, broadly effective therapeutic strategy for treating fungal infectious disease that evades resistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Brown, G.D. et al. Hidden killers: human fungal infections. Sci. Transl. Med. 4, 165rv13 (2012).
Shapiro, R.S., Robbins, N. & Cowen, L.E. Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol. Mol. Biol. Rev. 75, 213–267 (2011).
Denning, D.W. & Bromley, M.J. Infectious disease. How to bolster the antifungal pipeline. Science 347, 1414–1416 (2015).
Anderson, T.M. et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat. Chem. Biol. 10, 400–406 (2014).
Cowen, L.E. The fungal Achilles' heel: targeting Hsp90 to cripple fungal pathogens. Curr. Opin. Microbiol. 16, 377–384 (2013).
Leach, M.D., Klipp, E., Cowen, L.E. & Brown, A.J.P. Fungal Hsp90: a biological transistor that tunes cellular outputs to thermal inputs. Nat. Rev. Microbiol. 10, 693–704 (2012).
Cowen, L.E. et al. Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc. Natl. Acad. Sci. USA 106, 2818–2823 (2009).
Robbins, N. et al. Hsp90 governs dispersion and drug resistance of fungal biofilms. PLoS Pathog. 7, e1002257 (2011).
Cowen, L.E. & Lindquist, S. Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science 309, 2185–2189 (2005).
Singh, S.D. et al. Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin. PLoS Pathog. 5, e1000532 (2009).
Steinbach, W.J., Reedy, J.L., Cramer, R.A. Jr., Perfect, J.R. & Heitman, J. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat. Rev. Microbiol. 5, 418–430 (2007).
Zhang, L. et al. High-throughput synergy screening identifies microbial metabolites as combination agents for the treatment of fungal infections. Proc. Natl. Acad. Sci. USA 104, 4606–4611 (2007).
Heitman, J., Movva, N.R. & Hall, M.N. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 253, 905–909 (1991).
Suzuki, Y. et al. Knocking out multigene redundancies via cycles of sexual assortment and fluorescence selection. Nat. Methods 8, 159–164 (2011).
Prasad, R. & Goffeau, A. Yeast ATP-binding cassette transporters conferring multidrug resistance. Annu. Rev. Microbiol. 66, 39–63 (2012).
Gupta, R.P., Kueppers, P., Hanekop, N. & Schmitt, L. Generating symmetry in the asymmetric ATP-binding cassette (ABC) transporter Pdr5 from Saccharomyces cerevisiae. J. Biol. Chem. 289, 15272–15279 (2014).
Miyata, Y. Protein kinase CK2 in health and disease: CK2: the kinase controlling the Hsp90 chaperone machinery. Cell. Mol. Life Sci. 66, 1840–1849 (2009).
Kubiński, K. et al. Yeast holoenzyme of protein kinase CK2 requires both beta and beta' regulatory subunits for its activity. Mol. Cell. Biochem. 295, 229–236 (2007).
Teo, G. et al. SAINTexpress: improvements and additional features in Significance Analysis of INTeractome software. J. Proteomics 100, 37–43 (2014).
Martin, D.E. & Hall, M.N. The expanding TOR signaling network. Curr. Opin. Cell Biol. 17, 158–166 (2005).
Sanchez-Casalongue, M.E. et al. Differential phosphorylation of a regulatory subunit of protein kinase CK2 by target of rapamycin complex 1 signaling and the Cdc-like kinase Kns1. J. Biol. Chem. 290, 7221–7233 (2015).
Coccetti, P. et al. Mutations of the CK2 phosphorylation site of Sic1 affect cell size and S-Cdk kinase activity in Saccharomyces cerevisiae. Mol. Microbiol. 51, 447–460 (2004).
Coccetti, P. et al. Sic1 is phosphorylated by CK2 on Ser201 in budding yeast cells. Biochem. Biophys. Res. Commun. 346, 786–793 (2006).
Loewith, R. et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell 10, 457–468 (2002).
Cruz, M.C. et al. Rapamycin and less immunosuppressive analogs are toxic to Candida albicans and Cryptococcus neoformans via FKBP12-dependent inhibition of TOR. Antimicrob. Agents Chemother. 45, 3162–3170 (2001).
Cardenas, M.E., Cutler, N.S., Lorenz, M.C., Di Como, C.J. & Heitman, J. The TOR signaling cascade regulates gene expression in response to nutrients. Genes Dev. 13, 3271–3279 (1999).
Mollapour, M. et al. Threonine 22 phosphorylation attenuates Hsp90 interaction with cochaperones and affects its chaperone activity. Mol. Cell 41, 672–681 (2011).
Diezmann, S., Michaut, M., Shapiro, R.S., Bader, G.D. & Cowen, L.E. Mapping the Hsp90 genetic interaction network in Candida albicans reveals environmental contingency and rewired circuitry. PLoS Genet. 8, e1002562 (2012).
Leach, M.D. et al. Hsp90 orchestrates transcriptional regulation by Hsf1 and cell wall remodelling by MAPK signalling during thermal adaptation in a pathogenic yeast. PLoS Pathog. 8, e1003069 (2012).
Hawle, P. et al. Cdc37p is required for stress-induced high-osmolarity glycerol and protein kinase C mitogen-activated protein kinase pathway functionality by interaction with Hog1p and Slt2p (Mpk1p). Eukaryot. Cell 6, 521–532 (2007).
Stathopoulos, A.M. & Cyert, M.S. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 11, 3432–3444 (1997).
Shapiro, R.S. et al. Hsp90 orchestrates temperature-dependent Candida albicans morphogenesis via Ras1-PKA signaling. Curr. Biol. 19, 621–629 (2009).
Fukuda, T., Arai, M., Tomoda, H. & Omura, S. New beauvericins, potentiators of antifungal miconazole activity, Produced by Beauveria sp. FKI-1366. II. Structure elucidation. J. Antibiot. (Tokyo) 57, 117–124 (2004).
Arrowsmith, C.H. et al. The promise and peril of chemical probes. Nat. Chem. Biol. 11, 536–541 (2015).
Jacinto, E. & Hall, M.N. Tor signalling in bugs, brain and brawn. Nat. Rev. Mol. Cell Biol. 4, 117–126 (2003).
Bruno, V.M. & Mitchell, A.P. Regulation of azole drug susceptibility by Candida albicans protein kinase CK2. Mol. Microbiol. 56, 559–573 (2005).
Egner, R., Rosenthal, F.E., Kralli, A., Sanglard, D. & Kuchler, K. Genetic separation of FK506 susceptibility and drug transport in the yeast Pdr5 ATP-binding cassette multidrug resistance transporter. Mol. Biol. Cell 9, 523–543 (1998).
Ma, J.F., Grant, G. & Melera, P.W. Mutations in the sixth transmembrane domain of P-glycoprotein that alter the pattern of cross-resistance also alter sensitivity to cyclosporin A reversal. Mol. Pharmacol. 51, 922–930 (1997).
Katzmann, D.J., Epping, E.A. & Moye-Rowley, W.S. Mutational disruption of plasma membrane trafficking of Saccharomyces cerevisiae Yor1p, a homologue of mammalian multidrug resistance protein. Mol. Cell. Biol. 19, 2998–3009 (1999).
Roemer, T. et al. Confronting the challenges of natural product-based antifungal discovery. Chem. Biol. 18, 148–164 (2011).
Piper, P.W. & Millson, S.H. Spotlight on the microbes that produce heat shock protein 90-targeting antibiotics. Open Biol. 2, 120138 (2012).
Xu, Y. et al. Biosynthesis of the cyclooligomer depsipeptide beauvericin, a virulence factor of the entomopathogenic fungus Beauveria bassiana. Chem. Biol. 15, 898–907 (2008).
Lehár, J. et al. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat. Biotechnol. 27, 659–666 (2009).
Kumar, R., Musiyenko, A. & Barik, S. Plasmodium falciparum calcineurin and its association with heat shock protein 90: mechanisms for the antimalarial activity of cyclosporin A and synergism with geldanamycin. Mol. Biochem. Parasitol. 141, 29–37 (2005).
Barquilla, A., Crespo, J.L. & Navarro, M. Rapamycin inhibits trypanosome cell growth by preventing TOR complex 2 formation. Proc. Natl. Acad. Sci. USA 105, 14579–14584 (2008).
Wang, Q. & Xu, L. Beauvericin, a bioactive compound produced by fungi: a short review. Molecules 17, 2367–2377 (2012).
Hill, J.A., Ammar, R., Torti, D., Nislow, C. & Cowen, L.E. Genetic and genomic architecture of the evolution of resistance to antifungal drug combinations. PLoS Genet. 9, e1003390 (2013).
Cibulskis, K. et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat. Biotechnol. 31, 213–219 (2013).
Kolaczkowski, M. et al. Anticancer drugs, ionophoric peptides, and steroids as substrates of the yeast multidrug transporter Pdr5p. J. Biol. Chem. 271, 31543–31548 (1996).
Ernst, R. et al. A mutation of the H-loop selectively affects rhodamine transport by the yeast multidrug ABC transporter Pdr5. Proc. Natl. Acad. Sci. USA 105, 5069–5074 (2008).
Goffeau, A. & Dufour, J.P. Plasma membrane ATPase from the yeast Saccharomyces cerevisiae. Methods Enzymol. 157, 528–533 (1988).
Tripodi, F. et al. CK2 activity is modulated by growth rate in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 398, 44–50 (2010).
Liu, G. et al. ProHits: integrated software for mass spectrometry-based interaction proteomics. Nat. Biotechnol. 28, 1015–1017 (2010).
Choi, H. et al. SAINT: probabilistic scoring of affinity purification-mass spectrometry data. Nat. Methods 8, 70–73 (2011).
Choi, H. et al. Analyzing protein-protein interactions from affinity purification-mass spectrometry data with SAINT. Curr. Protoc. Bioinformatics Chapter 8 Unit8.15 (2012).
Urban, J. et al. Sch9 is a major target of TORC1 in Saccharomyces cerevisiae. Mol. Cell 26, 663–674 (2007).
Andes, D. et al. Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. Infect. Immun. 72, 6023–6031 (2004).
Acknowledgements
We thank the Donnelly Sequencing Centre for whole genome sequencing; the 2013 MGY360H1 class for performing and analyzing whole-genome sequencing data for a subset of the resistant mutants in the ABC16 background; D. Kim for assistance with genome sequencing analysis of the resistant mutants in the yor1Δ background; and all members of the Cowen lab for discussions. T.S.-G. is supported by the Ontario Graduate Scholarship and University of Toronto Open Fellowship. L.E.C. is supported by the Canadian Institutes of Health Research (CIHR) Operating Grants (MOP-86452 and MOP-119520), the Natural Sciences and Engineering Research Council (NSERC) of Canada Discovery Grants (06261 and 462167), an NSERC E.W.R. Steacie Memorial Fellowship (477598), and a Canada Research Chair in Microbial Genomics and Infectious Disease. J.H. is supported by the DUKE PO1 (AI104533-01) and RO1 (AI112595-02) for antifungal drug discovery. Y.-S.B is supported by the General International Collaborative R&D program funded by Ministry of Trade, Industry and Energy (MOTIE) in Republic of Korea (N0001720). A.A.L.G. is supported by US Institutes of Health Grants (R01 CA090265 and P41 GM094060). A.-C.G. is supported by a CIHR Foundation Grant, Genome Canada Genomics Innovation Network (GIN) Node and Technical Development Grants, and a Canada Research Chair in Functional Proteomics. J.-P.L was supported by a postdoctoral fellowship from the CIHR and by a TD Bank Health Research Fellowship at the Lunenfeld–Tanenbaum Research Institute. P.C. is supported by the Italian Government (FA). F.T. is supported by a postdoctoral fellowship from MIUR.
Author information
Authors and Affiliations
Contributions
L.E.C. and T.S.-G. conceived of and designed the study. T.S.-G. designed and performed selection experiments, drug-susceptibility assays, qRT-PCR, β-galactosidase activity assays, and western blots. R.A. and C.N. analyzed whole-genome sequencing data for a subset of the beauvericin-resistant strains in the ABC16 background. F.P.R. analyzed whole-genome sequencing data for a subset of the beauvericin-resistant strains in the ABC16 background. D.S. designed and performed the azole accumulation experiment. L.S. and K.D. designed and performed the in vitro Pdr5 ATPase and rhodamine 6G transport assays. G.M.K.B.G., E.M.K.W., and A.A.L.G. synthesized the biotinylated beauvericin analog. A.-C.G. and J.-P.L. designed the AP–MS experiment; J.-P.L. performed and analyzed AP–MS data under supervision of A.-C.G. F.T. and P.C. designed and performed CK2 kinase activity assays. R.J.L. and K.N.-S. designed and performed experiments to monitor TORC1 and TORC2 activity via Sch9 and Ypk1 phosphorylation, respectively. Y.-S.B., A.F.A., and J.H. designed the C. albicans mouse study; Y.-S.B., A.F.A., S.C.L., T.K., and J.H. performed and analyzed the mouse study. D.A. designed and performed the C. albicans biofilm study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Tables 1–4 and Supplementary Figures 1–12. (PDF 14000 kb)
Supplementary Note 1
Isolation of beauvericin (1) and synthesis of biotinyloxybeauvericin (PDF 600 kb)
Supplementary Note 2
Plasmid and strain construction (PDF 320 kb)
Rights and permissions
About this article
Cite this article
Shekhar-Guturja, T., Gunaherath, G., Wijeratne, E. et al. Dual action antifungal small molecule modulates multidrug efflux and TOR signaling. Nat Chem Biol 12, 867–875 (2016). https://doi.org/10.1038/nchembio.2165
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2165
This article is cited by
-
Molecular mechanisms governing antifungal drug resistance
npj Antimicrobials and Resistance (2023)
-
A small molecule produced by Lactobacillus species blocks Candida albicans filamentation by inhibiting a DYRK1-family kinase
Nature Communications (2021)
-
An oxindole efflux inhibitor potentiates azoles and impairs virulence in the fungal pathogen Candida auris
Nature Communications (2020)
-
Role of protein kinase CK2 in antitumor drug resistance
Journal of Experimental & Clinical Cancer Research (2019)
-
In vitro NTPase activity of highly purified Pdr5, a major yeast ABC multidrug transporter
Scientific Reports (2019)