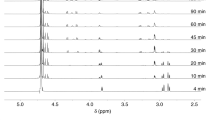
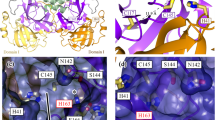
Abstract
Oxidation of methionine disrupts the structure and function of a range of proteins, but little is understood about the chemistry that underlies these perturbations. Using quantum mechanical calculations, we found that oxidation increased the strength of the methionine-aromatic interaction motif, a driving force for protein folding and protein-protein interaction, by 0.5–1.4 kcal/mol. We found that non-hydrogen-bonded interactions between dimethyl sulfoxide (a methionine analog) and aromatic groups were enriched in both the Protein Data Bank and Cambridge Structural Database. Thermal denaturation and NMR spectroscopy experiments on model peptides demonstrated that oxidation of methionine stabilized the interaction by 0.5–0.6 kcal/mol. We confirmed the biological relevance of these findings through a combination of cell biology, electron paramagnetic resonance spectroscopy and molecular dynamics simulations on (i) calmodulin structure and dynamics, and (ii) lymphotoxin-α binding toTNFR1. Thus, the methionine-aromatic motif was a determinant of protein structural and functional sensitivity to oxidative stress.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Valley, C.C. et al. The methionine-aromatic motif plays a unique role in stabilizing protein structure. J. Biol. Chem. 287, 34979–34991 (2012).
Zauhar, R.J., Colbert, C.L., Morgan, R.S. & Welsh, W.J. Evidence for a strong sulfur-aromatic interaction derived from crystallographic data. Biopolymers 53, 233–248 (2000).
Reid, K.S.C., Lindley, P.F. & Thornton, J.M. Sulphur-aromatic interactions in proteins. FEBS Lett. 190, 209–213 (1985).
Pranata, J. Sulfur–Aromatic Interactions: A Computational Study of the Dimethyl Sulfide–Benzene Complex. Bioorg. Chem. 25, 213–219 (1997).
Morgan, R.S. & McAdon, J.M. Predictor for sulfur-aromatic interactions in globular proteins. Int. J. Pept. Protein Res. 15, 177–180 (1980).
Morgan, R.S., Tatsch, C.E., Gushard, R.H., McAdon, J. & Warme, P.K. Chains of alternating sulfur and pi-bonded atoms in eight small proteins. Int. J. Pept. Protein Res. 11, 209–217 (1978).
Viguera, A.R. & Serrano, L. Side-chain interactions between sulfur-containing amino acids and phenylalanine in α-helices. Biochemistry 34, 8771–8779 (1995).
Shechter, Y., Burstein, Y. & Patchornik, A. Selective oxidation of methionine residues in proteins. Biochemistry 14, 4497–4503 (1975).
Hoshi, T. & Heinemann, S. Regulation of cell function by methionine oxidation and reduction. J. Physiol. (Lond.) 531, 1–11 (2001).
Levine, R.L., Berlett, B.S., Moskovitz, J., Mosoni, L. & Stadtman, E.R. Methionine residues may protect proteins from critical oxidative damage. Mech. Ageing Dev. 107, 323–332 (1999).
Kim, G., Weiss, S.J. & Levine, R.L. Methionine oxidation and reduction in proteins. Biochim. Biophys. Acta 1840, 901–905 (2014).
Arakawa, T., Kita, Y. & Timasheff, S.N. Protein precipitation and denaturation by dimethyl sulfoxide. Biophys. Chem. 131, 62–70 (2007).
Rose, G.D., Geselowitz, A.R., Lesser, G.J., Lee, R.H. & Zehfus, M.H. Hydrophobicity of amino acid residues in globular proteins. Science 229, 834–838 (1985).
Dougherty, D.A. Cation-pi interactions in chemistry and biology: a new view of benzene, Phe, Tyr, and Trp. Science 271, 163–168 (1996).
Bogan, A.A. & Thorn, K.S. Anatomy of hot spots in protein interfaces. J. Mol. Biol. 280, 1–9 (1998).
Gottlieb, H.E., Kotlyar, V. & Nudelman, A. NMR chemical shifts of common laboratory solvents as trace impurities. J. Org. Chem. 62, 7512–7515 (1997).
Butterfield, S.M., Patel, P.R. & Waters, M.L. Contribution of aromatic interactions to alpha-helix stability. J. Am. Chem. Soc. 124, 9751–9755 (2002).
Greenfield, N.J. Using circular dichroism collected as a function of temperature to determine the thermodynamics of protein unfolding and binding interactions. Nat. Protoc. 1, 2527–2535 (2006).
Marqusee, S. & Baldwin, R.L. Helix stabilization by Glu-...Lys+ salt bridges in short peptides of de novo design. Proc. Natl. Acad. Sci. USA 84, 8898–8902 (1987).
Pearson, K. LIII. On lines and planes of closest fit to systems of points in space. Phil. Mag. 2, 559–572 (1901).
Yin, D., Kuczera, K. & Squier, T.C. The sensitivity of carboxyl-terminal methionines in calmodulin isoforms to oxidation by H(2)O(2) modulates the ability to activate the plasma membrane Ca-ATPase. Chem. Res. Toxicol. 13, 103–110 (2000).
Sacksteder, C.A. et al. Tertiary structural rearrangements upon oxidation of Methionine145 in calmodulin promotes targeted proteasomal degradation. Biophys. J. 91, 1480–1493 (2006).
Gao, J., Yao, Y. & Squier, T.C. Oxidatively modified calmodulin binds to the plasma membrane Ca-ATPase in a nonproductive and conformationally disordered complex. Biophys. J. 80, 1791–1801 (2001).
Gao, J. et al. Loss of conformational stability in calmodulin upon methionine oxidation. Biophys. J. 74, 1115–1134 (1998).
Boschek, C.B., Jones, T.E., Smallwood, H.S., Squier, T.C. & Bigelow, D.J. Loss of the calmodulin-dependent inhibition of the RyR1 calcium release channel upon oxidation of methionines in calmodulin. Biochemistry 47, 131–142 (2008).
Bartlett, R.K. et al. Oxidation of Met144 and Met145 in calmodulin blocks calmodulin dependent activation of the plasma membrane Ca-ATPase. Biochemistry 42, 3231–3238 (2003).
Babu, Y.S., Bugg, C.E. & Cook, W.J. Structure of calmodulin refined at 2.2 A resolution. J. Mol. Biol. 204, 191–204 (1988).
Kuboniwa, H. et al. Solution structure of calcium-free calmodulin. Nat. Struct. Biol. 2, 768–776 (1995).
Mukherjea, P., Maune, J.F. & Beckingham, K. Interlobe communication in multiple calcium-binding site mutants of Drosophila calmodulin. Protein Sci. 5, 468–477 (1996).
Anbanandam, A. et al. Mediating molecular recognition by methionine oxidation: conformational switching by oxidation of methionine in the carboxyl-terminal domain of calmodulin. Biochemistry 44, 9486–9496 (2005).
Jeschke, G. DEER distance measurements on proteins. Annu. Rev. Phys. Chem. 63, 419–446 (2012).
Ilardi, E.A., Vitaku, E. & Njardarson, J.T. Data-mining for sulfur and fluorine: an evaluation of pharmaceuticals to reveal opportunities for drug design and discovery. J. Med. Chem. 57, 2832–2842 (2014).
Scott, J., Asami, M. & Tanaka, K. Novel chromogenic, guest-sensitive host compounds. New J. Chem. 26, 1822–1826 (2002).
Jiménez, M.A., Muñoz, V., Rico, M. & Serrano, L. Helix stop and start signals in peptides and proteins. The capping box does not necessarily prevent helix elongation. J. Mol. Biol. 242, 487–496 (1994).
Vasquez, M., Pincus, M.R. & Scheraga, H.A. Helix-coil transition theory including long-range electrostatic interactions: application to globular proteins. Biopolymers 26, 351–371 (1987).
Fealey, M.E. et al. Negative coupling as a mechanism for signal propagation between C2 domains of synaptotagmin I. PLoS One 7, e46748 (2012).
Horovitz, A. Double-mutant cycles: a powerful tool for analyzing protein structure and function. Fold. Des. 1, R121–R126 (1996).
Horovitz, A., Serrano, L., Avron, B., Bycroft, M. & Fersht, A.R. Strength and co-operativity of contributions of surface salt bridges to protein stability. J. Mol. Biol. 216, 1031–1044 (1990).
Serrano, L., Bycroft, M. & Fersht, A.R. Aromatic-aromatic interactions and protein stability. Investigation by double-mutant cycles. J. Mol. Biol. 218, 465–475 (1991).
Balog, E.M., Lockamy, E.L., Thomas, D.D. & Ferrington, D.A. Site-specific methionine oxidation initiates calmodulin degradation by the 20S proteasome. Biochemistry 48, 3005–3016 (2009).
Klein, J.C. et al. Actin-binding cleft closure in myosin II probed by site-directed spin labeling and pulsed EPR. Proc. Natl. Acad. Sci. USA 105, 12867–12872 (2008).
Etemadi, N. et al. Lymphotoxin α induces apoptosis, necroptosis and inflammatory signals with the same potency as tumour necrosis factor. FEBS J. 280, 5283–5297 (2013).
Frisch, M.J. et al. Gaussian 09, Revision D.01. (Gaussian, Inc., 2009).
Boys, S.F. & Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 19, 553–566 (1970).
Zhao, Y. & Truhlar, D.G. A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J. Chem. Phys. 125, 194101 (2006).
Reed, A.E., Curtiss, L.A. & Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 88, 899–926 (1988).
Mulliken, R.S. Electronic population analysis on LCAO–MO molecular wave functions. I. J. Chem. Phys. 23, 1833–1840 (1955).
Cramer, C.J. Essentials of Computational Chemistry: Theories and Models (John Wiley & Sons, 2013).
Phillips, J.C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005).
Banner, D.W. et al. Crystal structure of the soluble human 55 kd TNF receptor-human TNF beta complex: implications for TNF receptor activation. Cell 73, 431–445 (1993).
Jorgensen, W.L., Chandrasekhar, J., Madura, J.D., Impey, R.W. & Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
MacKerell, A.D. et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 102, 3586–3616 (1998).
MacKerell, A.D. Jr., Feig, M. & Brooks, C.L. III. Improved treatment of the protein backbone in empirical force fields. J. Am. Chem. Soc. 126, 698–699 (2004).
Best, R.B. et al. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone ϕ, ψ and side-chain χ(1) and χ(2) dihedral angles. J. Chem. Theory Comput. 8, 3257–3273 (2012).
Martyna, G.J., Tobias, D.J. & Klein, M.L. Constant-pressure molecular-dynamics algorithms. J. Chem. Phys. 101, 4177–4189 (1994).
Jas, G.S. & Kuczera, K. Free-energy simulations of the oxidation of c-terminal methionines in calmodulin. Proteins 48, 257–268 (2002).
Darden, T., Perera, L., Li, L. & Pedersen, L. New tricks for modelers from the crystallography toolkit: the particle mesh Ewald algorithm and its use in nucleic acid simulations. Structure 7, R55–R60 (1999).
Tuckerman, M., Berne, B.J. & Martyna, G.J. Reversible multiple time scale molecular dynamics. J. Chem. Phys. 97, 1990–2001 (1992).
Andersen, H.C. Rattle: a velocity version of the shake algorithm for molecular-dynamics calculations. J. Comput. Phys. 52, 24–34 (1983).
Acknowledgements
This work was supported by grants to J.N.S. (US National Institutes of Health (NIH) R01 GM107175), D.D.T. (NIH R37 AG26160), W.C.K.P. (US National Science Foundation (NSF)-CAREER CHE-1352091) and A.H. (NSF-CAREER MCB-0845676). This work was carried out in part using computing resources at the University of Minnesota Supercomputing Institute. EPR spectroscopy and CD experiments were performed at the Biophysical Spectroscopy Center, University of Minnesota. We thank J.F. Evans for discussion and guidance regarding the principal component analysis of our CD data.
Author information
Authors and Affiliations
Contributions
Project conception and production was directed by J.N.S. PDB search, molecular dynamics simulations, experimental and computational LTα work, and computational CaM work were performed by A.K.L., T.L.S., C.C.V. and J.N.S. CSD search and NMR spectroscopy were performed by G.T.P. and W.C.K.P. Quantum calculations were performed by M.A.J., A.C. and J.G. CD was performed by R.M., B.T.H., K.M.D. and A.H. Peptides were synthesized by C.B.K. CaM work was carried out by M.R.M, C.H. and D.D.T.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Tables 1–7 and Supplementary Figures 1–17. (PDF 2035 kb)
Rights and permissions
About this article
Cite this article
Lewis, A., Dunleavy, K., Senkow, T. et al. Oxidation increases the strength of the methionine-aromatic interaction. Nat Chem Biol 12, 860–866 (2016). https://doi.org/10.1038/nchembio.2159
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2159
This article is cited by
-
Heterotypic interactions can drive selective co-condensation of prion-like low-complexity domains of FET proteins and mammalian SWI/SNF complex
Nature Communications (2024)
-
Low dose DMSO treatment induces oligomerization and accelerates aggregation of α-synuclein
Scientific Reports (2022)
-
Role of DegQ in differential stability of flagellin subunits in Vibrio vulnificus
npj Biofilms and Microbiomes (2021)
-
Met125 is essential for maintaining the structural integrity of calmodulin’s C-terminal domain
Scientific Reports (2020)
-
Evolution of metazoan oxygen-sensing involved a conserved divergence of VHL affinity for HIF1α and HIF2α
Nature Communications (2019)