Abstract
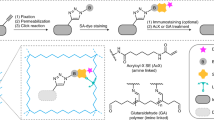
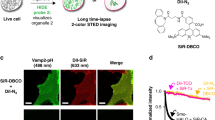
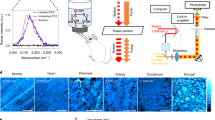
EM has long been the main technique for imaging cell structures with nanometer resolution but has lagged behind light microscopy in the crucial ability to make specific molecules stand out. Here we introduce click-EM, a labeling technique for correlative light microscopy and EM imaging of nonprotein biomolecules. In this approach, metabolic labeling substrates containing bioorthogonal functional groups are provided to cells for incorporation into biopolymers by endogenous biosynthetic machinery. The unique chemical functionality of these analogs is exploited for selective attachment of singlet oxygen-generating fluorescent dyes via bioorthogonal 'click chemistry' ligations. Illumination of dye-labeled structures generates singlet oxygen to locally catalyze the polymerization of diaminobenzidine into an osmiophilic reaction product that is readily imaged by EM. We describe the application of click-EM in imaging metabolically tagged DNA, RNA and lipids in cultured cells and neurons and highlight its use in tracking peptidoglycan synthesis in the Gram-positive bacterium Listeria monocytogenes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Stirling, J.W. Immuno- and affinity probes for electron microscopy: a review of labeling and preparation techniques. J. Histochem. Cytochem. 38, 145–157 (1990).
Deerinck, T.J. et al. Fluorescence photooxidation with eosin: a method for high resolution immunolocalization and in situ hybridization detection for light and electron microscopy. J. Cell Biol. 126, 901–910 (1994).
Shu, X. et al. A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS Biol. 9, e1001041 (2011).
Gaietta, G. et al. Multicolor and electron microscopic imaging of connexin trafficking. Science 296, 503–507 (2002).
Martell, J.D. et al. Engineered ascorbate peroxidase as a genetically encoded reporter for electron microscopy. Nat. Biotechnol. 30, 1143–1148 (2012).
Lam, S.S. et al. Directed evolution of APEX2 for electron microscopy and proximity labeling. Nat. Methods 12, 51–54 (2015).
Tyagi, S. Imaging intracellular RNA distribution and dynamics in living cells. Nat. Methods 6, 331–338 (2009).
Laughlin, S.T., Baskin, J.M., Amacher, S.L. & Bertozzi, C.R. In vivo imaging of membrane-associated glycans in developing zebrafish. Science 320, 664–667 (2008).
Salic, A. & Mitchison, T.J. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc. Natl. Acad. Sci. USA 105, 2415–2420 (2008).
Jao, C.Y. & Salic, A. Exploring RNA transcription and turnover in vivo by using click chemistry. Proc. Natl. Acad. Sci. USA 105, 15779–15784 (2008).
Jao, C.Y., Roth, M., Welti, R. & Salic, A. Metabolic labeling and direct imaging of choline phospholipids in vivo. Proc. Natl. Acad. Sci. USA 106, 15332–15337 (2009).
Ngo, J.T. & Tirrell, D.A. Noncanonical amino acids in the interrogation of cellular protein synthesis. Acc. Chem. Res. 44, 677–685 (2011).
Rostovtsev, V.V., Green, L.G., Fokin, V.V. & Sharpless, K.B. A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Edn Engl. 41, 2596–2599 (2002).
Tornøe, C.W., Christensen, C. & Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 67, 3057–3064 (2002).
Wang, Q. et al. Bioconjugation by copper(I)-catalyzed azide-alkyne [3 + 2] cycloaddition. J. Am. Chem. Soc. 125, 3192–3193 (2003).
Agard, N.J., Prescher, J.A. & Bertozzi, C.R. A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc. 126, 15046–15047 (2004).
Jewett, J.C. & Bertozzi, C.R. Cu-free click cycloaddition reactions in chemical biology. Chem. Soc. Rev. 39, 1272–1279 (2010).
Gandin, E., Lion, Y. & Van de Vorst, A. Quantum yield of singlet oxygen production by xanthene derivatives. Photochem. Photobiol. 37, 271–278 (1983).
Usui, Y. Determination of quantum yield of singlet oxygen formation by photosensitization. Chem. Lett. 7, 743–744 (1973).
Kishimoto, S. et al. Evaluation of oxygen dependence on in vitro and in vivo cytotoxicity of photoimmunotherapy using IR-700-antibody conjugates. Free Radic. Biol. Med. 85, 24–32 (2015).
Capani, F. et al. Phalloidin-eosin followed by photo-oxidation: a novel method for localizing F-actin at the light and electron microscopic levels. J. Histochem. Cytochem. 49, 1351–1361 (2001).
Chibisov, A.K., Zakharova, G.V. & Görner, H. Effects of substituents in the polymethine chain on the photoprocesses in indodicarbocyanine dyes. J. Chem. Soc., Faraday Trans. 92, 4917–4925 (1996).
Görner, H. & Chibisov, A.K. in CRC Handbook of Organic Photochemistry and Photobiology (eds. Horspool, W. & Lenci, F.) 36.1–36.21 (2004).
Velapoldi, R.A. & Tønnesen, H.H. Corrected emission spectra and quantum yields for a series of fluorescent compounds in the visible spectral region. J. Fluoresc. 14, 465–472 (2004).
Fleming, G.R., Knight, A.W.E., Morris, J.M., Morrison, R.J.S. & Robinson, G.W. Picosecond fluorescence studies of xanthene dyes. J. Am. Chem. Soc. 99, 4306–4311 (1977).
Dimitrova, D.S. & Berezney, R. The spatiotemporal organization of DNA replication sites is identical in primary, immortalized and transformed mammalian cells. J. Cell Sci. 115, 4037–4051 (2002).
Chagin, V.O., Stear, J.H. & Cardoso, M.C. Organization of DNA replication. Cold Spring Harb. Perspect. Biol. 2, a000737 (2010).
Iborra, F.J., Pombo, A., Jackson, D.A. & Cook, P.R. Active RNA polymerases are localized within discrete transcription “factories” in human nuclei. J. Cell Sci. 109, 1427–1436 (1996).
Papantonis, A. & Cook, P.R. Transcription factories: genome organization and gene regulation. Chem. Rev. 113, 8683–8705 (2013).
Bensaude, O. Inhibiting eukaryotic transcription: Which compound to choose? How to evaluate its activity? Transcription 2, 103–108 (2011).
Li, C. et al. Practical labeling methodology for choline-derived lipids and applications in live cell fluorescence imaging. Photochem. Photobiol. 90, 686–695 (2014).
Debets, M.F. et al. Aza-dibenzocyclooctynes for fast and efficient enzyme PEGylation via copper-free (3+2) cycloaddition. Chem. Commun. (Camb.) 46, 97–99 (2010).
van Meer, G., Voelker, D.R. & Feigenson, G.W. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9, 112–124 (2008).
Siegrist, M.S. et al. D-Amino acid chemical reporters reveal peptidoglycan dynamics of an intracellular pathogen. ACS Chem. Biol. 8, 500–505 (2013).
Cava, F., de Pedro, M.A., Lam, H., Davis, B.M. & Waldor, M.K. Distinct pathways for modification of the bacterial cell wall by non-canonical D-amino acids. EMBO J. 30, 3442–3453 (2011).
Siegrist, M.S., Swarts, B.M., Fox, D.M., Lim, S.A. & Bertozzi, C.R. Illumination of growth, division and secretion by metabolic labeling of the bacterial cell surface. FEMS Microbiol. Rev. 39, 184–202 (2015).
Pittman, J.R. et al. Effect of stressors on the viability of listeria during an in vitro cold-smoking process. Agric. Food Anal. Bacteriol. 2, 195–208 (2012).
Korsak, D., Vollmer, W. & Markiewicz, Z. Listeria monocytogenes EGD lacking penicillin-binding protein 5 (PBP5) produces a thicker cell wall. FEMS Microbiol. Lett. 251, 281–288 (2005).
Fang, X. et al. The mechanism of action of ramoplanin and enduracidin. Mol. Biosyst. 2, 69–76 (2006).
Littau, V.C., Allfrey, V.G., Frenster, J.H. & Mirsky, A.E. Active and inactive regions of nuclear chromatin as revealed by electron microscope autoradiography. Proc. Natl. Acad. Sci. USA 52, 93–100 (1964).
Gupta, B.L., Moreton, R.B. & Cooper, N.C. Reconsideration of the resolution in EM autoradiography using a biological line source. J. Microsc. 99, 1–25 (1973).
Iborra, F.J. & Cook, P.R. The size of sites containing SR proteins in human nuclei. Problems associated with characterizing small structures by immunogold labeling. J. Histochem. Cytochem. 46, 985–992 (1998).
Paez-Segala, M.G. et al. Fixation-resistant photoactivatable fluorescent proteins for CLEM. Nat. Methods 12, 215–218, 4, 218 (2015).
Perkovic, M. et al. Correlative light- and electron microscopy with chemical tags. J. Struct. Biol. 186, 205–213 (2014).
Devaraj, N.K., Hilderbrand, S., Upadhyay, R., Mazitschek, R. & Weissleder, R. Bioorthogonal turn-on probes for imaging small molecules inside living cells. Angew. Chem. Int. Edn Engl. 49, 2869–2872 (2010).
Shieh, P. et al. CalFluors: a universal motif for fluorogenic azide probes across the visible spectrum. J. Am. Chem. Soc. 137, 7145–7151 (2015).
Sanman, L.E. & Bogyo, M. Activity-based profiling of proteases. Annu. Rev. Biochem. 83, 249–273 (2014).
tom Dieck, S. et al. Direct visualization of newly synthesized target proteins in situ. Nat. Methods 12, 411–414 (2015).
Robinson, P.V., de Almeida-Escobedo, G., de Groot, A.E., McKechnie, J.L. & Bertozzi, C.R. Live-cell labeling of specific protein glycoforms by proximity-enhanced bioorthogonal ligation. J. Am. Chem. Soc. 137, 10452–10455 (2015).
Dieterich, D.C. et al. In situ visualization and dynamics of newly synthesized proteins in rat hippocampal neurons. Nat. Neurosci. 13, 897–905 (2010).
Bowers, P.G. & Porter, G. Triplet state quantum yields for some aromatic hydrocarbons and xanthene dyes in dilute solution. Proc. R. Soc. Lond. A 299, 348–353 (1967).
Pal, P. et al. Phototoxicity of some bromine-substituted rhodamine dyes: synthesis, photophysical properties and application as photosensitizers. Photochem. Photobiol. 63, 161–168 (1996).
Mujumdar, R.B., Ernst, L.A., Mujumdar, S.R., Lewis, C.J., & Waggoner, A.S. Cyanine dye labeling reagents: Sulfoindocyanine succinimidyl esters. Bioconj. Chem. 4, 105–111 (1993).
Caputo, G. & Della, C.L. Symmetric, monofunctionalised polymethine dyes labelling reagents. European patent 1221465A1 (2002).
Acknowledgements
We gratefully acknowledge S. Phan for assistance with SBEM image processing, C. Woodford for assistance with NMR, L. Gross for assistance with MS and C. Hill for providing wild-type and lmo2754::tn EGD-e L. monocytogenes. Funding for this work was provided by NIH GM086197 (to R.Y.T. and M.H.E.), NIH GM058867 (to C.R.B.), and NIH AI051622 (to C.R.B.). The work described herein was carried out using shared research resources at the National Center for Microscopy and Imaging Research (NCMIR) at UCSD supported by the NIH under award number P41 GM103412 (to M.H.E.). F.R. was supported by a Ford Foundation Predoctoral Fellowship and a Chancellor's fellowship from UC Berkeley.
Author information
Authors and Affiliations
Contributions
J.T.N., S.R.A., T.J.D., D.B., F.R.-R. and S.F.P. performed experiments. J.T.N., S.R.A., T.J.D., D.B., F.R.-R., S.F.P., C.R.B., M.H.E. and R.Y.T. analyzed data and prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results and Supplementary Figures 1–10. (PDF 12247 kb)
Rights and permissions
About this article
Cite this article
Ngo, J., Adams, S., Deerinck, T. et al. Click-EM for imaging metabolically tagged nonprotein biomolecules. Nat Chem Biol 12, 459–465 (2016). https://doi.org/10.1038/nchembio.2076
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2076
This article is cited by
-
Proximal Molecular Probe Transfer (PROMPT), a new approach for identifying sites of protein/nucleic acid interaction in cells by correlated light and electron microscopy
Scientific Reports (2023)
-
Chromatin reprogramming and bone regeneration in vitro and in vivo via the microtopography-induced constriction of cell nuclei
Nature Biomedical Engineering (2023)
-
Parallel gold enhancement of quantum dots 565/655 for double-labelling correlative light and electron microscopy on human autopsied samples
Scientific Reports (2022)
-
In vivo 5-ethynyluridine (EU) labelling detects reduced transcription in Purkinje cell degeneration mouse mutants, but can itself induce neurodegeneration
Acta Neuropathologica Communications (2021)
-
Bioorthogonal chemistry
Nature Reviews Methods Primers (2021)