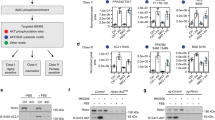
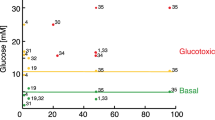
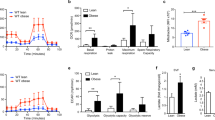
Abstract
The critical cellular hydride donor NADPH is produced through various means, including the oxidative pentose phosphate pathway (oxPPP), folate metabolism and malic enzyme. In growing cells, it is efficient to produce NADPH via the oxPPP and folate metabolism, which also make nucleotide precursors. In nonproliferating adipocytes, a metabolic cycle involving malic enzyme holds the potential to make both NADPH and two-carbon units for fat synthesis. Recently developed deuterium (2H) tracer methods have enabled direct measurement of NADPH production by the oxPPP and folate metabolism. Here we enable tracking of NADPH production by malic enzyme with [2,2,3,3-2H]dimethyl-succinate and [4-2H]glucose. Using these tracers, we show that most NADPH in differentiating 3T3-L1 mouse adipocytes is made by malic enzyme. The associated metabolic cycle is disrupted by hypoxia, which switches the main adipocyte NADPH source to the oxPPP. Thus, 2H-labeled tracers enable dissection of NADPH production routes across cell types and environmental conditions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Voet, D. & Voet, J. Biochemistry 3rd edn. (Wiley, 2004).
Tibbetts, A.S. & Appling, D.R. Compartmentalization of mammalian folate-mediated one-carbon metabolism. Annu. Rev. Nutr. 30, 57–81 (2010).
Wise, D.R. et al. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of α-ketoglutarate to citrate to support cell growth and viability. Proc. Natl. Acad. Sci. USA 108, 19611–19616 (2011).
WHO Working Group. Glucose-6-phosphate dehydrogenase deficiency. Bull. World Health Organ. 67, 601–611 (1989).
Fan, J. et al. Quantitative flux analysis reveals folate-dependent NADPH production. Nature 510, 298–302 (2014).
Lewis, C.A. et al. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol. Cell 55, 253–263 (2014).
Nguyen, P. et al. Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. (Berl.) 92, 272–283 (2008).
Young, J.W., Shrago, E. & Lardy, H.A. Metabolic control of enzymes involved in lipogenesis and gluconeogenesis. Biochemistry 3, 1687–1692 (1964).
Wise, E.M. Jr. & Ball, E.G. Malic enzyme and lipogenesis. Proc. Natl. Acad. Sci. USA 52, 1255–1263 (1964).
Wise, L.S., Sul, H.S. & Rubin, C.S. Coordinate regulation of the biosynthesis of ATP-citrate lyase and malic enzyme during adipocyte differentiation. Studies on 3T3-L1 cells. J. Biol. Chem. 259, 4827–4832 (1984).
Si, Y., Yoon, J. & Lee, K. Flux profile and modularity analysis of time-dependent metabolic changes of de novo adipocyte formation. Am. J. Physiol. Endocrinol. Metab. 292, E1637–E1646 (2007).
Katz, J. & Rognstad, R. The metabolism of tritiated glucose by rat adipose tissue. J. Biol. Chem. 241, 3600–3610 (1966).
Kather, H., Rivera, M. & Brand, K. Interrelationship and control of glucose metabolism and lipogenesis in isolated fat cells. Control of pentose phosphate-cycle activity by cellular requirement for reduced nicotinamide adenine dinucleotide phosphate. Biochem. J. 128, 1097–1102 (1972).
Flatt, J.P. & Ball, E.G. Studies on the metabolism of adipose tissue: XV. An evaluation of the major pathways of glucose catabolism as influenced by insulin and epinephrine on the metabolism of adipose. J. Biol. Chem. 239, 675–685 (1964).
Green, H. & Meuth, M. An established pre-adipose cell line and its differentiation in culture. Cell 3, 127–133 (1974).
Rosen, E.D. & MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 7, 885–896 (2006).
Shreve, D.S. & Levy, H.R. Kinetic mechanism of glucose-6-phosphate dehydrogenase from the lactating rat mammary gland. Implications for regulation. J. Biol. Chem. 255, 2670–2677 (1980).
Yang, X.M. & MacKenzie, R.E. NAD-dependent methylenetetrahydrofolate dehydrogenase-methenyltetrahydrofolate cyclohydrolase is the mammalian homolog of the mitochondrial enzyme encoded by the yeast MIS1 gene. Biochemistry 32, 11118–11123 (1993).
Ochoa, S., Mehler, A.H. & Kornberg, A. Biosynthesis of dicarboxylic acids by carbon dioxide fixation; isolation and properties of an enzyme from pigeon liver catalyzing the reversible oxidative decarboxylation of 1-malic acid. J. Biol. Chem. 174, 979–1000 (1948).
Jitrapakdee, S. et al. Structure, mechanism and regulation of pyruvate carboxylase. Biochem. J. 413, 369–387 (2008).
Rutter, W.J. & Lardy, H.A. Purification and properties of pigeon liver malic enzyme. J. Biol. Chem. 233, 374–382 (1958).
DeBerardinis, R.J. et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA 104, 19345–19350 (2007).
Yuan, Z. & Hammes, G.G. Elementary steps in the reaction mechanism of chicken liver fatty acid synthase. pH dependence of NADPH binding and isotope rate effect for beta-ketoacyl reductase. J. Biol. Chem. 259, 6748–6751 (1984).
Jiang, P., Du, W., Mancuso, A., Wellen, K.E. & Yang, X. Reciprocal regulation of p53 and malic enzymes modulates metabolism and senescence. Nature 493, 689–693 (2013).
Si, Y., Shi, H. & Lee, K. Impact of perturbed pyruvate metabolism on adipocyte triglyceride accumulation. Metab. Eng. 11, 382–390 (2009).
Kim, J.W., Tchernyshyov, I., Semenza, G.L. & Dang, C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 3, 177–185 (2006).
Lu, C.W., Lin, S.C., Chen, K.F., Lai, Y.Y. & Tsai, S.J. Induction of pyruvate dehydrogenase kinase-3 by hypoxia-inducible factor-1 promotes metabolic switch and drug resistance. J. Biol. Chem. 283, 28106–28114 (2008).
Hosogai, N. et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56, 901–911 (2007).
Trayhurn, P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol. Rev. 93, 1–21 (2013).
Mullen, A.R. et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 481, 385–388 (2012).
Kamphorst, J.J., Chung, M.K., Fan, J. & Rabinowitz, J.D. Quantitative analysis of acetyl-CoA production in hypoxic cancer cells reveals substantial contribution from acetate. Cancer Metab. 2, 23 (2014).
Price, N.E. & Cook, P.F. Kinetic and chemical mechanisms of the sheep liver 6-phosphogluconate dehydrogenase. Arch. Biochem. Biophys. 336, 215–223 (1996).
Hermes, J.D., Roeske, C.A., O'Leary, M.H. & Cleland, W.W. Use of multiple isotope effects to determine enzyme mechanisms and intrinsic isotope effects. Malic enzyme and glucose-6-phosphate dehydrogenase. Biochemistry 21, 5106–5114 (1982).
Al-Dwairi, A., Pabona, J.M.P., Simmen, R.C.M. & Simmen, F.A. Cytosolic malic enzyme 1 (ME1) mediates high fat diet-induced adiposity, endocrine profile, and gastrointestinal tract proliferation-associated biomarkers in male mice. PLoS ONE 7, e46716 (2012).
Lee, C.Y., Lee, S.M., Lewis, S. & Johnson, F.M. Identification and biochemical analysis of mouse mutants deficient in cytoplasmic malic enzyme. Biochemistry 19, 5098–5103 (1980).
Koh, H.-J. et al. Cytosolic NADP+-dependent isocitrate dehydrogenase plays a key role in lipid metabolism. J. Biol. Chem. 279, 39968–39974 (2004).
Wellen, K.E. et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324, 1076–1080 (2009).
Boucher, A. et al. Biochemical mechanism of lipid-induced impairment of glucose-stimulated insulin secretion and reversal with a malate analogue. J. Biol. Chem. 279, 27263–27271 (2004).
Munger, J. et al. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat. Biotechnol. 26, 1179–1186 (2008).
Lu, W. et al. Metabolomic analysis via reversed-phase ion-pairing liquid chromatography coupled to a stand alone orbitrap mass spectrometer. Anal. Chem. 82, 3212–3221 (2010).
Sutterlin, H.A., Zhang, S. & Silhavy, T.J. Accumulation of phosphatidic acid increases vancomycin resistance in Escherichia coli. J. Bacteriol. 196, 3214–3220 (2014).
Edens, W.A., Urbauer, J.L. & Cleland, W.W. Determination of the chemical mechanism of malic enzyme by isotope effects. Biochemistry 36, 1141–1147 (1997).
Seyama, Y. et al. Identification of sources of hydrogen atoms in fatty acids synthesized using deuterated water and stereospecifically deuterium labelled NADPH by gas chromatographic mass spectrometric analysis. Biomed. Mass Spectrom. 5, 357–361 (1978).
Wiechert, W., Möllney, M., Isermann, N., Wurzel, M. & de Graaf, A.A. Bidirectional reaction steps in metabolic networks: III. Explicit solution and analysis of isotopomer labeling systems. Biotechnol. Bioeng. 66, 69–85 (1999).
Weitzel, M. et al. 13CFLUX2—high-performance software suite for 13C metabolic flux analysis. Bioinformatics 29, 143–145 (2013).
Waltz, R.A., Morales, J.L., Nocedal, J. & Orban, D. An interior algorithm for nonlinear optimization that combines line search and trust region steps. Math. Program. 107, 391–408 (2006).
Antoniewicz, M.R., Kelleher, J.K. & Stephanopoulos, G. Determination of confidence intervals of metabolic fluxes estimated from stable isotope measurements. Metab. Eng. 8, 324–337 (2006).
Acknowledgements
We thank C. Thompson and M. Birnbaum for helpful discussions. This work was supported by US National Institutes of Health grants R01CA163591 (J.D.R.), R01AI097382 (J.D.R.) and P30DK019525 (to the University of Pennsylvania Diabetes Research Center). J.F. was supported by a Howard Hughes Medical Institute fellowship. K.E.W. is supported by American Diabetes Association grant 7-12-JF-59. S.S. is supported by postdoctoral fellowship 5T32CA009140-40.
Author information
Authors and Affiliations
Contributions
J.D.R., K.E.W., L.L. and J.F. conceived the project. L.L. performed and analyzed most experiments. L.L., J.O.P., J.F. and J.D.R. conducted the flux analysis. S.S. performed electroporation experiments and analyzed the data. J.D.R. and L.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Figures 1–13 and Supplementary Tables 1 and 2. (PDF 10153 kb)
Rights and permissions
About this article
Cite this article
Liu, L., Shah, S., Fan, J. et al. Malic enzyme tracers reveal hypoxia-induced switch in adipocyte NADPH pathway usage. Nat Chem Biol 12, 345–352 (2016). https://doi.org/10.1038/nchembio.2047
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2047
This article is cited by
-
AKT1 phosphorylation of cytoplasmic ME2 induces a metabolic switch to glycolysis for tumorigenesis
Nature Communications (2024)
-
Cytosolic and mitochondrial NADPH fluxes are independently regulated
Nature Chemical Biology (2023)
-
The pentose phosphate pathway in health and disease
Nature Metabolism (2023)
-
IDH2, a novel target of OGT, facilitates glucose uptake and cellular bioenergy production via NF-κB signaling to promote colorectal cancer progression
Cellular Oncology (2023)
-
Switching to the cyclic pentose phosphate pathway powers the oxidative burst in activated neutrophils
Nature Metabolism (2022)