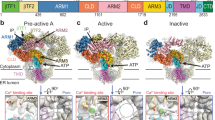
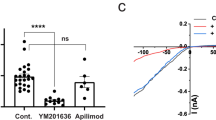
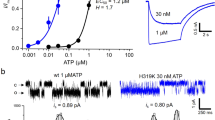
Abstract
Inositol 1,4,5-trisphosphate receptors (IP3Rs) are ubiquitous intracellular Ca2+ channels. IP3 binding to the IP3-binding core (IBC) near the N terminus initiates conformational changes that lead to opening of a pore. The mechanisms underlying this process are unresolved. We synthesized 2-O–modified IP3 analogs that are partial agonists of IP3R. These are similar to IP3 in their interactions with the IBC, but they are less effective than IP3 in rearranging the relationship between the IBC and the N-terminal suppressor domain (SD), and they open the channel at slower rates. IP3R with a mutation in the SD occupying a position similar to the 2-O substituent of the partial agonists has a reduced open probability that is similar for full and partial agonists. Bulky or charged substituents from either the ligand or the SD therefore block obligatory coupling of the IBC and the SD. Analysis of ΔG for ligand binding shows that IP3 is recognized by the IBC and conformational changes then propagate entirely via the SD to the pore.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Foskett, J.K., White, C., Cheung, K.H. & Mak, D.O. Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 87, 593–658 (2007).
MacKinnon, R. Potassium channels and the atomic basis of selective ion conduction (Nobel lecture). Angew. Chem. Int. Edn Engl. 43, 4265–4277 (2004).
Bosanac, I. et al. Structure of the inositol 1,4,5-trisphosphate receptor binding core in complex with its ligand. Nature 420, 696–700 (2002).
Uchida, K., Miyauchi, H., Furuichi, T., Michikawa, T. & Mikoshiba, K. Critical regions for activation gating of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 278, 16551–16560 (2003).
Szlufcik, K. et al. The suppressor domain of inositol 1,4,5-trisphosphate receptor plays an essential role in the protection against apoptosis. Cell Calcium 39, 325–336 (2006).
Taylor, C.W., da Fonseca, P.C.A. & Morris, E.P. IP3 receptors: the search for structure. Trends Biochem. Sci. 29, 210–219 (2004).
Lape, R., Colquhoun, D. & Sivilotti, L.G. On the nature of partial agonism in the nicotinic receptor superfamily. Nature 454, 722–727 (2008).
Auerbach, A. Gating of acetylcholine receptor channels: Brownian motion across a broad transition state. Proc. Natl. Acad. Sci. USA 102, 1408–1412 (2005).
Monod, J., Wyman, J. & Changeux, J.P. On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 12, 88–118 (1965).
Colquhoun, D. Binding, gating, affinity and efficacy. Br. J. Pharmacol. 125, 923–947 (1998).
Stephenson, R.P. A modification of receptor theory. Br. J. Pharmacol. 11, 379–393 (1956).
Jin, R., Banke, T.G., Mayer, M.L., Traynelis, S.F. & Gouax, E. Structural basis for partial agonist action at ionotropic glutamate receptors. Nat. Neurosci. 6, 803–810 (2003).
Mayer, M.L. Glutamate receptors at atomic resolution. Nature 440, 456–462 (2006).
Banke, T.G. & Traynelis, S.F. Activation of NR1/NR2B NMDA receptors. Nat. Neurosci. 6, 144–152 (2003).
Popescu, G. & Auerbach, A. Modal gating of NMDA receptors and the shape of their synaptic response. Nat. Neurosci. 6, 476–483 (2003).
Jencks, W.P. Binding energy, specificity, and enzymic catalysis: the circe effect. Adv. Enzymol. Relat. Areas Mol. Biol. 43, 219–410 (1975).
Burgen, A.S.V. Conformational changes and drug action. Fed. Proc. 40, 2723–2728 (1981).
Potter, B.V.L. & Lampe, D. Chemistry of inositol lipid mediated cellular signaling. Angew. Chem. Int. Edn Engl. 34, 1933–1972 (1995).
Riley, A.M., Laude, A.J., Taylor, C.W. & Potter, B.V.L. Dimers of D-myo-inositol 1,4,5-trisphosphate: design, synthesis, and interaction with Ins(1,4,5)P3 receptors. Bioconjug. Chem. 15, 278–289 (2004).
Riley, A.M. et al. Interactions of inositol 1,4,5-trisphosphate (IP3) receptors with synthetic poly(ethylene glycol)-linked dimers of IP3 suggest close spacing of IP3-binding sites. J. Biol. Chem. 277, 40290–40295 (2002).
Kramer, R.H. & Karpen, J.W. Spanning binding sites on allosteric proteins with polymer-linked ligand dimers. Nature 395, 710–713 (1998).
Poinas, A. et al. Study of the interaction of the catalytic domain of Ins(1,4,5)P3 3-kinase A with inositol phosphate analogues. ChemBioChem 6, 1449–1457 (2005).
Riley, A.M., Dozol, H., Spiess, B. & Potter, B.V.L. 2-O-(2-aminoethyl)-myo-inositol 1,4,5-trisphosphate as a novel ligand for conjugation: physicochemical properties and synthesis of a new Ins(1,4,5)P3 affinity matrix. Biochem. Biophys. Res. Commun. 318, 444–452 (2004).
Tovey, S.C., Sun, Y. & Taylor, C.W. Rapid functional assays of intracellular Ca2+ channels. Nat. Protoc. 1, 258–262 (2006).
Takahashi, M., Tanzawa, K. & Takahashi, S. Adenophostins, newly discovered metabolites of Penicillium brevicompactum, act as potent agonists of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 269, 369–372 (1994).
Dellis, O. et al. Ca2+ entry through plasma membrane IP3 receptors. Science 313, 229–233 (2006).
Rahman, T.-U., Skupin, A., Falcke, M. & Taylor, C.W. Clustering of IP3 receptors by IP3 retunes their regulation by IP3 and Ca2+. Nature 458, 655–659 (2009).
Iwai, M., Michikawa, T., Bosanac, I., Ikura, M. & Mikoshiba, K. Molecular basis of the isoform-specific ligand-binding affinity of inositol 1,4,5-trisphosphate receptors. J. Biol. Chem. 282, 12755–12764 (2007).
Yoshikawa, F. et al. Mutational analysis of the ligand binding site of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 271, 18277–18284 (1996).
Williams, D.H., Zhou, M. & Stephens, E. Ligand binding energy and enzyme efficiency from reductions in protein dynamics. J. Mol. Biol. 355, 760–767 (2006).
Boehning, D. & Joseph, S.K. Direct association of ligand-binding and pore domains in homo- and heterotetrameric inositol 1,4,5-trisphosphate receptors. EMBO J. 19, 5450–5459 (2000).
Schug, Z.T. & Joseph, S.K. The role of the S4–S5 linker and C-terminal tail in inositol 1,4,5-trisphosphate receptor function. J. Biol. Chem. 281, 24431–24440 (2006).
Bosanac, I. et al. Crystal structure of the ligand binding suppressor domain of type 1 inositol 1,4,5-trisphosphate receptor. Mol. Cell 17, 193–203 (2005).
Chan, J. et al. Ligand-induced conformational changes via flexible linkers in the amino-terminal region of the inositol 1,4,5-trisphosphate receptor. J. Mol. Biol. 373, 1269–1280 (2007).
Kobilka, B.K. & Deupi, X. Conformational complexity of G-protein-coupled receptors. Trends Pharmacol. Sci. 28, 397–406 (2007).
Bosanac, I., Michikawa, T., Mikoshiba, K. & Ikura, M. Structural insights into the regulatory mechanism of IP3 receptor. Biochim. Biophys. Acta 1742, 89–102 (2004).
George, C.H., Jundi, H., Thomas, N.L., Fry, D.L. & Lai, F.A. Ryanodine receptors and ventricular arrhythmias: emerging trends in mutations, mechanisms and therapies. J. Mol. Cell. Cardiol. 42, 34–50 (2007).
Wagenknecht, T. & Samsó, M. Three-dimensional reconstruction of ryanodine receptors. Front. Biosci. 7, 1464–1474 (2002).
Marwood, R.D., Correa, V., Taylor, C.W. & Potter, B.V.L. Synthesis of adenophostin A. Tetrahedron Asymmetry 11, 397–403 (2000).
Riley, A.M. et al. Scyllo-inositol pentakisphosphate as an analogue of myo-inositol 1,3,4,5,6-pentakisphosphate: chemical synthesis, physicochemistry and biological applications. ChemBioChem 7, 1114–1122 (2006).
Sugawara, H., Kurosaki, M., Takata, M. & Kurosaki, T. Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. EMBO J. 16, 3078–3088 (1997).
Cardy, T.J.A., Traynor, D. & Taylor, C.W. Differential regulation of types 1 and 3 inositol trisphosphate receptors by cytosolic Ca2+. Biochem. J. 328, 785–793 (1997).
Yoshikawa, F. et al. High efficient expression of the functional ligand binding site of the inositol 1,4,5-trisphosphate receptor in Escherichia coli. Biochem. Biophys. Res. Commun. 257, 792–797 (1999).
Jiang, Q.-X., Thrower, E.C., Chester, D.W., Ehrlich, B.E. & Sigworth, F.J. Three-dimensional structure of the type 1 inositol 1,4,5-trisphosphate receptor at 24 Å resolution. EMBO J. 21, 3575–3581 (2002).
Colquhoun, D. Lectures in Biostatistics 279–343 (Clarendon Press, Oxford, 1971).
Ritchie, D.W., Kozakov, D. & Vajda, S. Accelerating and focusing protein-protein docking correlations using multi-dimensional rotational FFT generating functions. Bioinformatics 24, 1865–1873 (2008).
Gray, J.J. et al. Protein-protein docking with simultaneous optimization of rigid-body displacement and side-chain conformations. J. Mol. Biol. 331, 281–299 (2003).
Riley, A.M., Correa, V., Mahon, M.F., Taylor, C.W. & Potter, B.V.L. Bicyclic analogues of D-myo-inositol 1,4,5-trisphosphate related to adenophostin A: synthesis and biological activity. J. Med. Chem. 44, 2108–2117 (2001).
Riley, A.M., Guédat, P., Schlewer, G., Spiess, B. & Potter, B.V.L. A conformationally restricted cyclic phosphate analogue of inositol trisphosphate: synthesis and physicochemical properties. J. Org. Chem. 63, 295–305 (1998).
Riley, A.M. & Potter, B.V.L. Poly(ethylene glycol)-linked dimers of D-myo-inositol 1,4,5-trisphosphate. Chem. Commun. (Camb.) 983–984 (2000).
Acknowledgements
We thank S. Dedos, P. da Fonseca, A. Burgen, S. Otto and M. Garcia Alai for helpful comments, and T. Woodman for advice on NMR spectroscopy. This work was supported by grants from the Wellcome Trust (to C.W.T., A.M. Riley and B.V.L.P.) and the Biotechnology and Biological Sciences Research Council (to C.W.T.). A.M. Rossi holds a Junior Research Fellowship at Queens' College, Cambridge, UK.
Author information
Authors and Affiliations
Contributions
A.M. Rossi, S.C.T., T.R., O.D. and E.J.A.T. completed the biology experiments. V.G.V. performed molecular modeling. A.M. Riley designed and synthesized the ligands and contributed to molecular modeling. B.V.L.P. (chemistry) and C.W.T. (biology) designed and coordinated the project. C.W.T. and A.M. Rossi wrote the manuscript with input from the other authors. All authors discussed the results and commented on the manuscript.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4, Supplementary Tables 1–6 and Supplementary Methods (PDF 1264 kb)
Rights and permissions
About this article
Cite this article
Rossi, A., Riley, A., Tovey, S. et al. Synthetic partial agonists reveal key steps in IP3 receptor activation. Nat Chem Biol 5, 631–639 (2009). https://doi.org/10.1038/nchembio.195
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.195
This article is cited by
-
Exploration of inositol 1,4,5-trisphosphate (IP3) regulated dynamics of N-terminal domain of IP3 receptor reveals early phase molecular events during receptor activation
Scientific Reports (2019)
-
Cryo-EM reveals ligand induced allostery underlying InsP3R channel gating
Cell Research (2018)
-
A novel gain-of-function mutation in the ITPR1 suppressor domain causes spinocerebellar ataxia with altered Ca2+ signal patterns
Journal of Neurology (2017)
-
Structural characterization of human heparanase reveals insights into substrate recognition
Nature Structural & Molecular Biology (2015)
-
Structural and functional conservation of key domains in InsP3 and ryanodine receptors
Nature (2012)