Abstract
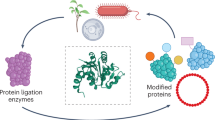
Current methods for engineering enzymes modify enzymes themselves and require a detailed mechanistic understanding or a high-throughput assay. Here, we describe a new approach where catalytic properties are modulated with synthetic binding proteins, termed monobodies, directed to an unmodified enzyme. Using the example of a β-galactosidase from Bacillus circulans, we efficiently identified monobodies that restricted its substrates for its transgalactosylation reaction and selectively enhanced the production of small oligosaccharide prebiotics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Wilks, H.M. et al. Science 242, 1541–1544 (1988).
Chen, K.Q. & Arnold, F.H. BioTechnology 9, 1073–1077 (1991).
Bornscheuer, U.T. et al. Nature 485, 185–194 (2012).
Davids, T., Schmidt, M., Bottcher, D. & Bornscheuer, U.T. Curr. Opin. Chem. Biol. 17, 215–220 (2013).
Goldsmith, M. & Tawfik, D.S. Curr. Opin. Struct. Biol. 22, 406–412 (2012).
Mak, W.S. & Siegel, J.B. Curr. Opin. Struct. Biol. 27, 87–94 (2014).
Song, J. et al. Biosci. Biotechnol. Biochem. 75, 1194–1197 (2011).
Barile, D. & Rastall, R.A. Curr. Opin. Biotechnol. 24, 214–219 (2013).
Bultema, J.B., Kuipers, B.J. & Dijkhuizen, L. FEBS Open Bio 4, 1015–1020 (2014).
Song, J. et al. Biosci. Biotechnol. Biochem. 75, 268–278 (2011).
Ishikawa, K. et al. FEBS J. 282, 2540–2552 (2015).
Strokopytov, B. et al. Biochemistry 34, 2234–2240 (1995).
Vocadlo, D.J., Davies, G.J., Laine, R. & Withers, S.G. Nature 412, 835–838 (2001).
Blake, C.C. et al. Proc. R. Soc. Lond. B Biol. Sci. 167, 378–388 (1967).
Davies, G.J., Wilson, K.S. & Henrissat, B. Biochem. J. 321, 557–559 (1997).
van der Veen, B.A. et al. J. Mol. Biol. 296, 1027–1038 (2000).
Chen-Goodspeed, M., Sogorb, M.A., Wu, F. & Raushel, F.M. Biochemistry 40, 1332–1339 (2001).
Schmitt, J., Brocca, S., Schmid, R.D. & Pleiss, J. Protein Eng. 15, 595–601 (2002).
Koide, A., Bailey, C.W., Huang, X. & Koide, S. J. Mol. Biol. 284, 1141–1151 (1998).
Koide, A., Wojcik, J., Gilbreth, R.N., Hoey, R.J. & Koide, S. J. Mol. Biol. 415, 393–405 (2012).
Wojcik, J. et al. Nat. Struct. Mol. Biol. 17, 519–527 (2010).
Gilbreth, R.N. et al. Proc. Natl. Acad. Sci. USA 108, 7751–7756 (2011).
Sha, F. et al. Proc. Natl. Acad. Sci. USA 110, 14924–14929 (2013).
Albayrak, N. & Yang, S.T. Biotechnol. Bioeng. 77, 8–19 (2002).
Li, S., Yang, X., Yang, S., Zhu, M. & Wang, X. Comput Struct. Biotechnol. J. 2, e201209017 (2012).
Koide, A., Gilbreth, R.N., Esaki, K., Tereshko, V. & Koide, S. Proc. Natl. Acad. Sci. USA 104, 6632–6637 (2007).
Larkin, M.A. et al. Bioinformatics 23, 2947–2948 (2007).
Goujon, M. et al. Nucleic Acids Res. 38, W695–W699 (2010).
Koide, S., Koide, A. & Lipovsek, D. Methods Enzymol. 503, 135–156 (2012).
Nishikori, S. et al. J. Mol. Biol. 424, 391–399 (2012).
Acknowledgements
We acknowledge the use of the University of Chicago Genomics Core, which is supported by US National Institutes of Health grant P30CA014599 to the University of Chicago Comprehensive Cancer Center.
Author information
Authors and Affiliations
Contributions
S.T. and S. Koide designed the research. S.T. and A.K. generated and characterized the monobodies. S.T. and T.T. analyzed effects of the monobodies on enzyme reactions. T.T. and S.I. conducted mutagenesis analysis of BgaD. S. Koikeda and S. Koide supervised the research. S.T. and S. Koide wrote the manuscript and the other authors commented on it.
Corresponding author
Ethics declarations
Competing interests
S. Tanaka, T. Takahashi, S. Ishihara and S. Koikeda are employees of Amano Enzyme that manufactures the Biolacta enzyme. S. Tanaka, T. Takahashi and S. Koikeda are shareholders of Amano Enzyme. The University of Chicago has filed a patent application on technologies described in this paper.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Figures 1–8 and Supplementary Table 1. (PDF 2143 kb)
Rights and permissions
About this article
Cite this article
Tanaka, Si., Takahashi, T., Koide, A. et al. Monobody-mediated alteration of enzyme specificity. Nat Chem Biol 11, 762–764 (2015). https://doi.org/10.1038/nchembio.1896
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1896
This article is cited by
-
Tuning SAS-6 architecture with monobodies impairs distinct steps of centriole assembly
Nature Communications (2021)
-
Targeted rescue of cancer-associated IDH1 mutant activity using an engineered synthetic antibody
Scientific Reports (2017)