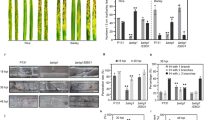
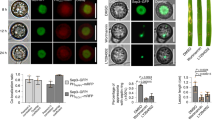
Abstract
Distinct modifications fine-tune the activity of jasmonic acid (JA) in regulating plant growth and immunity. Hydroxylated JA (12OH-JA) promotes flower and tuber development but prevents induction of JA signaling, plant defense or both. However, biosynthesis of 12OH-JA has remained elusive. We report here an antibiotic biosynthesis monooxygenase (Abm) that converts endogenous free JA into 12OH-JA in the model rice blast fungus Magnaporthe oryzae. Such fungal 12OH-JA is secreted during host penetration and helps evade the defense response. Loss of Abm in M. oryzae led to accumulation of methyl JA (MeJA), which induces host defense and blocks invasive growth. Exogenously added 12OH-JA markedly attenuated abmΔ-induced immunity in rice. Notably, Abm itself is secreted after invasion and most likely converts plant JA into 12OH-JA to facilitate host colonization. This study sheds light on the chemical arms race during plant-pathogen interaction, reveals Abm as an antifungal target and outlines a synthetic strategy for transformation of a versatile small-molecule phytohormone.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Loake, G. & Grant, M. Salicylic acid in plant defence—the players and protagonists. Curr. Opin. Plant Biol. 10, 466–472 (2007).
Pieterse, C.M., Leon-Reyes, A., Van der Ent, S. & Van Wees, S.C. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 5, 308–316 (2009).
Pozo, M.J., Van Loon, L.C. & Pieterse, C.M.J. Jasmonates—signals in plant-microbe interactions. J. Plant Growth Regul. 23, 211–222 (2004).
Felix, G. & Boller, T. Systemin induces rapid ion fluxes and ethylene biosynthesis in Lycopersicon peruvianum cells. Plant J. 7, 381–389 (1995).
Moyen, C. & Johannes, E. Systemin transiently depolarizes the tomato mesophyll cell membrane and antagonizes fusicoccin-induced extracellular acidification of mesophyll tissue. Plant Cell Environ. 19, 464–470 (1996).
Narvaez-Vasquez, J., Florin-Christensen, J. & Ryan, C.A. Positional specificity of a phospholipase A activity induced by wounding, systemin, and oligosaccharide elicitors in tomato leaves. Plant Cell 11, 2249–2260 (1999).
Król, P., Igielski, R., Pollmann, S. & Kepczynska, E. Priming of seeds with methyl jasmonate induced resistance to hemi-biotroph Fusarium oxysporum f. sp. lycopersici in tomato via 12-oxo-phytodienoic acid, salicylic acid, and flavonol accumulation. J. Plant Physiol. 179, 122–132 (2015).
Zhang, Y.T. et al. Proteomics of methyl jasmonate induced defense response in maize leaves against Asian corn borer. BMC Genomics 16, 224 (2015).
Gidda, S.K. et al. Biochemical and molecular characterization of a hydroxyjasmonate sulfotransferase from Arabidopsis thaliana. J. Biol. Chem. 278, 17895–17900 (2003).
Miersch, O., Neumerkel, J., Dippe, M., Stenzel, I. & Wasternack, C. Hydroxylated jasmonates are commonly occurring metabolites of jasmonic acid and contribute to a partial switch-off in jasmonate signaling. New Phytol. 177, 114–127 (2008).
Miersch, O., Bohlmann, H. & Wasternack, C. Jasmonates and related compounds from Fusarium oxysporum. Phytochemistry 50, 517–523 (1999).
Miersch, O., Porzel, A. & Wasternack, C. Microbial conversion of jasmonates-hydroxylations by Aspergillus niger. Phytochemistry 50, 1147–1152 (1999).
Yoshihara, T. et al. Structure of a tuber-inducing stimulus from potato leaves (Solanum tuberosum L.). Agr. Biol. Chem. Tokyo 53, 2835–2837 (1989).
Theoharides, A.D. & Kupfer, D. Evidence for different hepatic microsomal monooxygenases catalyzing omega- and (omega-1)-hydroxylations of prostaglandins E1 and E2. Effects of inducers of monooxygenase on the kinetic constants of prostaglandin hydroxylation. J. Biol. Chem. 256, 2168–2175 (1981).
Werck-Reichhart, D., Bak, S. & Paquette, S. Cytochromes P450. in The Arabidopsis Book/American Society of Plant Biologists, 1, e0028 (2002).
Cross, B.E. & Webster, G.R. New metabolites of Gibberella fujikuroi. XV. N-jasmonoyl- and N-dihydrojasmonoyl-isoleucine. J. Chem. Soc. Perk T1 13, 1839–1342 (1970).
Miersch, O., Gunther, T., Fritsche, W. & Sembdner, G. Jasmonates from different fungal species. Nat. Prod. Lett. 2, 293–299 (1993).
Miersch, O., Schneider, G. & Sembdner, G. Hydroxylated jasmonic acid and related compounds from Botryodiplodia theobromae. Phytochemistry 30, 4049–4051 (1991).
Brodhun, F. & Feussner, I. Oxylipins in fungi. FEBS J. 278, 1047–1063 (2011).
Dean, R. et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 13, 414–430 (2012).
Dayton, L. Agribiotechnology: blue-sky rice. Nature 514, S52–S54 (2014).
Riemann, M. et al. Identification of rice allene oxide cyclase mutants and the function of jasmonate for defence against Magnaporthe oryzae. Plant J. 74, 226–238 (2013).
Patkar, R.N., Xue, Y.K., Shui, G., Wenk, M.R. & Naqvi, N.I. Abc3-mediated efflux of an endogenous digoxin-like steroidal glycoside by Magnaporthe oryzae is necessary for host invasion during blast disease. PLoS Pathog. 8, e1002888 (2012).
Letelier, M.E. et al. Melatonin protects the cytochrome P450 system through a novel antioxidant mechanism. Chem. Biol. Interact. 185, 208–214 (2010).
Cucurou, C., Battioni, J.P., Thang, D.C., Nam, N.H. & Mansuy, D. Mechanisms of inactivation of lipoxygenases by phenidone and BW755C. Biochemistry 30, 8964–8970 (1991).
Radhika, V., Kost, C., Boland, W. & Heil, M. The role of jasmonates in floral nectar secretion. PLoS ONE 5, e9265 (2010).
Larrieu, A. et al. A fluorescent hormone biosensor reveals the dynamics of jasmonate signalling in plants. Nat. Commun. 6, 6043 (2015).
Giraldo, M.C. et al. Two distinct secretion systems facilitate tissue invasion by the rice blast fungus Magnaporthe oryzae. Nat. Commun. 4, 1996 (2013).
Grocholski, T. et al. Crystal structure of the cofactor-independent monooxygenase SnoaB from Streptomyces nogalater: implications for the reaction mechanism. Biochemistry 49, 934–944 (2010).
Snyder, B.A., Leite, B., Hipskind, J., Butler, L.G. & Nicholson, R.L. Accumulation of sorghum phytoalexins induced by Colletotrichum graminicola at the infection site. Physiol. Mol. Plant Pathol. 39, 463–470 (1991).
Horie, K. et al. Identification of UV-induced diterpenes including a new diterpene phytoalexin, pF, from rice leaves by complementary GC/MS and LC/MS approaches. J. Agric. Food Chem. 63, 4050–4059 (2015).
Umemura, K. et al. Possible role of phytocassane, rice phytoalexin, in disease resistance of rice against the blast fungus Magnaporthe grisea. Biosci. Biotechnol. Biochem. 67, 899–902 (2003).
Chi, M.H., Park, S.Y., Kim, S. & Lee, Y.H. A novel pathogenicity gene is required in the rice blast fungus to suppress the basal defenses of the host. PLoS Pathog. 5, e1000401 (2009).
Deng, Y.Z., Qu, Z., He, Y. & Naqvi, N.I. Sorting nexin Snx41 is essential for conidiation and mediates glutathione-based antioxidant defense during invasive growth in Magnaporthe oryzae. Autophagy 8, 1058–1070 (2012).
Fernandez, J. et al. Plant defence suppression is mediated by a fungal sirtuin during rice infection by Magnaporthe oryzae. Mol. Microbiol. 94, 70–88 (2014).
Huang, K., Czymmek, K.J., Caplan, J.L., Sweigard, J.A. & Donofrio, N.M. HYR1-mediated detoxification of reactive oxygen species is required for full virulence in the rice blast fungus. PLoS Pathog. 7, e1001335 (2011).
Schweizer, P. et al. Jasmonate-iducible genes are activated in rice by pathogen attack without a concomitant increase in endogenous jasmonic acid levels. Plant Physiol. 114, 79–88 (1997).
Schweizer, P., Gees, R. & Mosinger, E. Effect of jasmonic acid on the interaction of barley (Hordeum vulgare L.) with the powdery mildew Erysiphe graminis f.sp. hordei. Plant Physiol. 102, 503–511 (1993).
Schaller, F., Biesgen, C., Mussig, C., Altmann, T. & Weiler, E.W. 12-Oxophytodienoate reductase 3 (OPR3) is the isoenzyme involved in jasmonate biosynthesis. Planta 210, 979–984 (2000).
Weber, H. Fatty acid–derived signals in plants. Trends Plant Sci. 7, 217–224 (2002).
Theodoulou, F.L. et al. Jasmonic acid levels are reduced in COMATOSE ATP-binding cassette transporter mutants. Implications for transport of jasmonate precursors into peroxisomes. Plant Physiol. 137, 835–840 (2005).
Hedden, P. & Phillips, A.L. Manipulation of hormone biosynthetic genes in transgenic plants. Curr. Opin. Biotechnol. 11, 130–137 (2000).
Weber, H., Vick, B.A. & Farmer, E.E. Dinor-oxo-phytodienoic acid: a new hexadecanoid signal in the jasmonate family. Proc. Natl. Acad. Sci. USA 94, 10473–10478 (1997).
Taki, N. et al. 12-oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiol. 139, 1268–1283 (2005).
Park, J.W., Reed, J.R., Brignac-Huber, L.M. & Backes, W.L. Cytochrome P450 system proteins reside in different regions of the endoplasmic reticulum. Biochem. J. 464, 242–249 (2014).
Xin, X., Mains, R.E. & Eipper, B.A. Monooxygenase X, a member of the copper-dependent monooxygenase family localized to the endoplasmic reticulum. J. Biol. Chem. 279, 48159–48167 (2004).
Tanaka, S. et al. A secreted Ustilago maydis effector promotes virulence by targeting anthocyanin biosynthesis in maize. Elife 3, e01355 (2014).
Djamei, A. et al. Metabolic priming by a secreted fungal effector. Nature 478, 395–398 (2011).
Creelman, R.A. & Mullet, J.E. Jasmonic acid distribution and action in plants: regulation during development and response to biotic and abiotic stress. Proc. Natl. Acad. Sci. USA 92, 4114–4119 (1995).
Koda, Y. et al. Potato tuber inducing activities of jasmonic acid and related compounds. Phytochemistry 30, 1435–1438 (1991).
Patkar, R.N., Ramos-Pamplona, M., Gupta, A.P., Fan, Y. & Naqvi, N.I. Mitochondrial β-oxidation regulates organellar integrity and is necessary for conidial germination and invasive growth in Magnaporthe oryzae. Mol. Microbiol. 86, 1345–1363 (2012).
Patkar, R.N., Suresh, A. & Naqvi, N.I. MoTea4-mediated polarized growth is essential for proper asexual development and pathogenesis in Magnaporthe oryzae. Eukaryot. Cell 9, 1029–1038 (2010).
Yang, F. & Naqvi, N.I. Sulfonylurea resistance reconstitution as a novel strategy for ILV2-specific integration in Magnaporthe oryzae. Fungal Genet. Biol. 68, 71–76 (2014).
Khang, C.H. et al. Translocation of Magnaporthe oryzae effectors into rice cells and their subsequent cell-to-cell movement. Plant Cell 22, 1388–1403 (2010).
Valent, B., Farrall, L. & Chumley, F.G. Magnaporthe grisea genes for pathogenicity and virulence identified through a series of backcrosses. Genetics 127, 87–101 (1991).
Kankanala, P., Czymmek, K. & Valent, B. Roles for rice membrane dynamics and plasmodesmata during biotrophic invasion by the blast fungus. Plant Cell 19, 706–724 (2007).
Acknowledgements
We thank P. Staswick (University of Nebraska) for the chemically synthesized 12OH-JA and L. Laplaze (Institut de recherche pour le développement, France) for sharing Jas9-VENUS plasmids and transgenic Arabidopsis seeds. We thank L. Yixin and Z. Bo for help with ChemDraw. We also thank the Fungal Patho-Biology group for helpful discussions and suggestions. We are grateful to M. Calvert for help in spinning-disk confocal microscopy. This research was carried out using funds from the Temasek Life Sciences Laboratory (Singapore) and the National Research Foundation (Prime Minister's Office; NRF-CRP7-2010-02), Singapore.
Author information
Authors and Affiliations
Contributions
R.N.P. conceived, designed and performed the experiment, analyzed the data and co-wrote the manuscript. P.I.B. performed the chemical analyses and interpreted the data. Z.Q. performed the experiments. Y.Y.C.C. performed the initial gene-deletion analysis. F.Y. performed the experiments. S.S. contributed the analysis tools and interpreted and analyzed the data. N.I.N. conceived and designed the experiments; provided reagents, materials and analysis tools; analyzed and interpreted the data; and co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Table 1 and Supplementary Figures 1–15. (PDF 19870 kb)
41589_2015_BFnchembio1885_MOESM249_ESM.mov
Dynamics of Jas9-VENUS and H2B-RFP over a 30 min period in transgenic Arabidopsis roots treated with 1% ethanol (MOV 1649 kb)
41589_2015_BFnchembio1885_MOESM250_ESM.mov
Dynamics of Jas9-VENUS and H2B-RFP over a 30 min period in transgenic Arabidopsis roots treated with 50 μM JA (MOV 1528 kb)
41589_2015_BFnchembio1885_MOESM251_ESM.mov
Dynamics of Jas9-VENUS and H2B-RFP over a 30 min period in transgenic Arabidopsis roots treated with 50 μM MeJA (MOV 1156 kb)
41589_2015_BFnchembio1885_MOESM252_ESM.mov
Dynamics of Jas9-VENUS and H2B-RFP over a 30 min period in transgenic Arabidopsis roots treated with 50 μM 12OH-JA (MOV 1484 kb)
Rights and permissions
About this article
Cite this article
Patkar, R., Benke, P., Qu, Z. et al. A fungal monooxygenase-derived jasmonate attenuates host innate immunity. Nat Chem Biol 11, 733–740 (2015). https://doi.org/10.1038/nchembio.1885
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1885
This article is cited by
-
Jasmonic acid contributes to rice resistance against Magnaporthe oryzae
BMC Plant Biology (2022)
-
Evasion of plant immunity by microbial pathogens
Nature Reviews Microbiology (2022)
-
Characterization of infected process and primary mechanism in rice Acuce defense against rice blast fungus, Magnaporthe oryzae
Plant Molecular Biology (2022)
-
Feruloyl esterase Fae1 is required specifically for host colonisation by the rice-blast fungus Magnaporthe oryzae
Current Genetics (2022)
-
Arms and ammunitions: effectors at the interface of rice and it’s pathogens and pests
Rice (2021)