Abstract
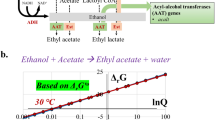
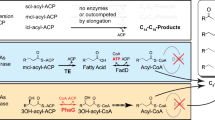
To expand the capabilities of whole-cell biocatalysis, we have engineered Escherichia coli to produce various esters. The alcohol O-acyltransferase (ATF) class of enzyme uses acyl-CoA units for ester formation. The release of free CoA upon esterification with an alcohol provides the free energy to facilitate ester formation. The diversity of CoA molecules found in nature in combination with various alcohol biosynthetic pathways allows for the biosynthesis of a multitude of esters. Small to medium volatile esters have extensive applications in the flavor, fragrance, cosmetic, solvent, paint and coating industries. The present work enables the production of these compounds by designing several ester pathways in E. coli. The engineered pathways generated acetate esters of ethyl, propyl, isobutyl, 2-methyl-1-butyl, 3-methyl-1-butyl and 2-phenylethyl alcohols. In particular, we achieved high-level production of isobutyl acetate from glucose (17.2 g l−1). This strategy was expanded to realize pathways for tetradecyl acetate and several isobutyrate esters.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Beekwilder, J. et al. Functional characterization of enzymes forming volatile esters from strawberry and banana. Plant Physiol. 135, 1865–1878 (2004).
Verstrepen, K.J. et al. Expression levels of the yeast alcohol acetyltransferase genes ATF1, Lg-ATF1, and ATF2 control the formation of a broad range of volatile esters. Appl. Environ. Microbiol. 69, 5228–5237 (2003).
Iwasaki, T., Maegawa, Y., Ohshima, T. & Mashima, K. in Kirk-Othmer Encyclopedia of Chemical Technology Vol. 10, 497–516 (John Wiley & Sons, New Jersey, 2012).
Liu, Y.J., Lotero, E. & Goodwin, J.G. Effect of water on sulfuric acid catalyzed esterification. J. Mol. Catal. Chem. 245, 132–140 (2006).
Nelson, D.L. & Cox, M.M. Lehninger Principles of Biochemistry 5th edn. (Sara Tenney, New York, 2008).
Stergiou, P.Y. et al. Advances in lipase-catalyzed esterification reactions. Biotechnol. Adv. 31, 1846–1859 (2013).
Dhake, K.P., Thakare, D.D. & Bhanage, B.M. Lipase: a potential biocatalyst for the synthesis of valuable flavour and fragrance ester compounds. Flavour Fragrance J. 28, 71–83 (2013).
Rabinovitch-Deere, C.A., Oliver, J.W., Rodriguez, G.M. & Atsumi, S. Synthetic biology and metabolic engineering approaches to produce biofuels. Chem. Rev. 113, 4611–4632 (2013).
Bornscheuer, U.T. et al. Engineering the third wave of biocatalysis. Nature 485, 185–194 (2012).
Fujii, T. et al. Molecular cloning, sequence analysis, and expression of the yeast alcohol acetyltransferase gene. Appl. Environ. Microbiol. 60, 2786–2792 (1994).
Rowland, O. & Domergue, F. Plant fatty acyl reductases: enzymes generating fatty alcohols for protective layers with potential for industrial applications. Plant Sci. 193–194, 28–38 (2012).
Rontani, J.F., Bonin, P.C. & Volkman, J.K. Production of wax esters during aerobic growth of marine bacteria on isoprenoid compounds. Appl. Environ. Microbiol. 65, 221–230 (1999).
Lenneman, E.M., Ohlert, J.M., Palani, N.P. & Barney, B.M. Fatty alcohols for wax esters in Marinobacter aquaeolei VT8: two optional routes in the wax biosynthesis pathway. Appl. Environ. Microbiol. 79, 7055–7062 (2013).
Reiser, S. & Somerville, C. Isolation of mutants of Acinetobacter calcoaceticus deficient in wax ester synthesis and complementation of one mutation with a gene encoding a fatty acyl coenzyme A reductase. J. Bacteriol. 179, 2969–2975 (1997).
Holtzapple, E. & Schmidt-Dannert, C. Biosynthesis of isoprenoid wax ester in Marinobacter hydrocarbonoclasticus DSM 8798: identification and characterization of isoprenoid coenzyme A synthetase and wax ester synthases. J. Bacteriol. 189, 3804–3812 (2007).
Barney, B.M., Wahlen, B.D., Garner, E., Wei, J. & Seefeldt, L.C. Differences in substrate specificities of five bacterial wax ester synthases. Appl. Environ. Microbiol. 78, 5734–5745 (2012).
Stöveken, T., Kalscheuer, R., Malkus, U., Reichelt, R. & Steinbuchel, A. The wax ester synthase/acyl coenzyme A:diacylglycerol acyltransferase from Acinetobacter sp. strain ADP1: characterization of a novel type of acyltransferase. J. Bacteriol. 187, 1369–1376 (2005).
Atsumi, S., Hanai, T. & Liao, J.C. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 451, 86–89 (2008).
Shen, C.R. et al. Driving forces enable high-titer anaerobic 1-butanol synthesis in Escherichia coli. Appl. Environ. Microbiol. 77, 2905–2915 (2011).
Bond-Watts, B.B., Bellerose, R.J. & Chang, M.C. Enzyme mechanism as a kinetic control element for designing synthetic biofuel pathways. Nat. Chem. Biol. 7, 222–227 (2011).
Inokuma, K., Liao, J.C., Okamoto, M. & Hanai, T. Improvement of isopropanol production by metabolically engineered Escherichia coli using gas stripping. J. Biosci. Bioeng. 110, 696–701 (2010).
Dellomonaco, C., Clomburg, J.M., Miller, E.N. & Gonzalez, R. Engineered reversal of the β-oxidation cycle for the synthesis of fuels and chemicals. Nature 476, 355–359 (2011).
Saerens, S.M. et al. The Saccharomyces cerevisiae EHT1 and EEB1 genes encode novel enzymes with medium-chain fatty acid ethyl ester synthesis and hydrolysis capacity. J. Biol. Chem. 281, 4446–4456 (2006).
Lilly, M. et al. The effect of increased yeast alcohol acetyltransferase and esterase activity on the flavour profiles of wine and distillates. Yeast 23, 641–659 (2006).
Mason, A.B. & Dufour, J.P. Alcohol acetyltransferases and the significance of ester synthesis in yeast. Yeast 16, 1287–1298 (2000).
Fujii, T., Yoshimoto, H. & Tamai, Y. Acetate ester production by Saccharomyces cerevisiae lacking the ATF1 gene encoding the alcohol acetyltransferase. J. Ferment. Bioeng. 81, 538–542 (1996).
Howard, T.P. et al. Synthesis of customized petroleum-replica fuel molecules by targeted modification of free fatty acid pools in Escherichia coli. Proc. Natl. Acad. Sci. USA 110, 7636–7641 (2013).
Lin, F., Das, D., Lin, X.N. & Marsh, E.N. Aldehyde-forming fatty acyl-CoA reductase from cyanobacteria: expression, purification and characterization of the recombinant enzyme. FEBS J. 280, 4773–4781 (2013).
Schirmer, A., Rude, M.A., Li, X., Popova, E. & del Cardayre, S.B. Microbial biosynthesis of alkanes. Science 329, 559–562 (2010).
Mooney, B.P., Miernyk, J.A. & Randall, D.D. The complex fate of α-ketoacids. Annu. Rev. Plant Biol. 53, 357–375 (2002).
Rodriguez, G.M. & Atsumi, S. Isobutyraldehyde production from Escherichia coli by removing aldehyde reductase activity. Microb. Cell Fact. 11, 90 (2012).
de la Plaza, M., Fernandez de Palencia, P., Pelaez, C. & Requena, T. Biochemical and molecular characterization of α-ketoisovalerate decarboxylase, an enzyme involved in the formation of aldehydes from amino acids by Lactococcus lactis. FEMS Microbiol. Lett. 238, 367–374 (2004).
Bastian, S. et al. Engineered ketol-acid reductoisomerase and alcohol dehydrogenase enable anaerobic 2-methylpropan-1-ol production at theoretical yield in Escherichia coli. Metab. Eng. 13, 345–352 (2011).
Baez, A., Cho, K.M. & Liao, J.C. High-flux isobutanol production using engineered Escherichia coli: a bioreactor study with in situ product removal. Appl. Microbiol. Biotechnol. 90, 1681–1690 (2011).
Atsumi, S. et al. Engineering the isobutanol biosynthetic pathway in Escherichia coli by comparison of three aldehyde reductase/alcohol dehydrogenase genes. Appl. Microbiol. Biotechnol. 85, 651–657 (2010).
Connor, M.R., Cann, A.F. & Liao, J.C. 3-Methyl-1-butanol production in Escherichia coli: random mutagenesis and two-phase fermentation. Appl. Microbiol. Biotechnol. 86, 1155–1164 (2010).
Belas, R. et al. Bacterial bioluminescence: isolation and expression of the luciferase genes from Vibrio harveyi. Science 218, 791–793 (1982).
Meighen, E.A. Bacterial bioluminescence: organization, regulation, and application of the lux genes. FASEB J. 7, 1016–1022 (1993).
Ferrández, A., Garcia, J.L. & Diaz, E. Genetic characterization and expression in heterologous hosts of the 3-(3-hydroxyphenyl)propionate catabolic pathway of Escherichia coli K-12. J. Bacteriol. 179, 2573–2581 (1997).
Lee, S.J., Ko, J.H., Kang, H.Y. & Lee, Y. Coupled expression of MhpE aldolase and MhpF dehydrogenase in Escherichia coli. Biochem. Biophys. Res. Commun. 346, 1009–1015 (2006).
Hester, K., Luo, J., Burns, G., Braswell, E.H. & Sokatch, J.R. Purification of active E1α2β2 of Pseudomonas putida branched-chain-oxoacid dehydrogenase. Eur. J. Biochem. 233, 828–836 (1995).
Hester, K.L., Luo, J. & Sokatch, J.R. Purification of Pseudonomas Putida branched-chain keto acid dehydrogenase E1 component. Methods Enzymol. 324, 129–138 (2000).
Alonso-Gutierrez, J. et al. Metabolic engineering of Escherichia coli for limonene and perillyl alcohol production. Metab. Eng. 19, 33–41 (2013).
Park, Y.C., Shaffer, C.E. & Bennett, G.N. Microbial formation of esters. Appl. Microbiol. Biotechnol. 85, 13–25 (2009).
van den Berg, C., Heeres, A.S., Van der Wielen, L.A. & Straathof, A.J. Simultaneous Clostridial fermentation, lipase-catalyzed esterification, and ester extraction to enrich diesel with butyl butyrate. Biotechnol. Bioeng. 110, 137–142 (2013).
Machado, H.B., Dekishima, Y., Luo, H., Lan, E.I. & Liao, J.C. A selection platform for carbon chain elongation using the CoA-dependent pathway to produce linear higher alcohols. Metab. Eng. 14, 504–511 (2012).
Acknowledgements
This work was supported by University of California–Davis startup fund and the Hellman fellowship to S.A. G.M.R. is supported by a US National Institutes of Health Biotechnology Training Grant Fellowship (T32-GM008799) and a Sloan Fellowship. Y.T. is supported by Japan Society for the Promotion of Science postdoctoral fellowship for research abroad. We would like to thank R. Luu and R.E. Parales (University of California–Davis) for graciously providing genomic DNA from P. putida g7 and M. Kato and S.-J. Lin (University of California–Davis) for providing genomic DNA from S. cerevisiae BY4742. We also thank M.D. Toney and C.A. Rabinovitch-Deere (University of California–Davis) for critical reading of the manuscript. Finally, we thank S. Desai for technical assistance with HPLC analysis and for constructing pAL603.
Author information
Authors and Affiliations
Contributions
G.M.R., Y.T. and S.A. designed research; G.M.R. and Y.T. performed the experiments; G.M.R., Y.T. and S.A. analyzed data; and G.M.R., Y.T. and S.A. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Tables 1–6 and Supplementary Figures 1–6. (PDF 1187 kb)
Rights and permissions
About this article
Cite this article
Rodriguez, G., Tashiro, Y. & Atsumi, S. Expanding ester biosynthesis in Escherichia coli. Nat Chem Biol 10, 259–265 (2014). https://doi.org/10.1038/nchembio.1476
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1476
This article is cited by
-
Microbial production of high octane and high sensitivity olefinic ester biofuels
Biotechnology for Biofuels and Bioproducts (2023)
-
Genome-wide analysis reveals Hsf1 maintains high transcript abundance of target genes controlled by strong constitutive promoter in Saccharomyces cerevisiae
Biotechnology for Biofuels and Bioproducts (2023)
-
Unravelling the hidden power of esterases for biomanufacturing of short-chain esters
Scientific Reports (2023)
-
Gas-phase stability and thermodynamics of ligand-bound, binary complexes of chloramphenicol acetyltransferase reveal negative cooperativity
Analytical and Bioanalytical Chemistry (2023)
-
Escherichia coli coculture for de novo production of esters derived of methyl-branched alcohols and multi-methyl branched fatty acids
Microbial Cell Factories (2022)