Abstract
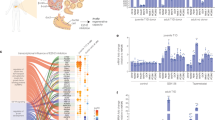
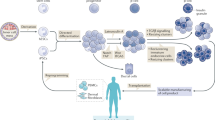
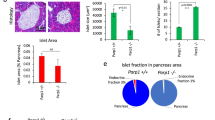
Cell replacement therapy for diabetes mellitus requires cost-effective generation of high-quality, insulin-producing, pancreatic β cells from pluripotent stem cells. Development of this technique has been hampered by a lack of knowledge of the molecular mechanisms underlying β-cell differentiation. The present study identified reserpine and tetrabenazine (TBZ), both vesicular monoamine transporter 2 (VMAT2) inhibitors, as promoters of late-stage differentiation of Pdx1-positive pancreatic progenitor cells into Neurog3 (referred to henceforth as Ngn3)-positive endocrine precursors. VMAT2-controlled monoamines, such as dopamine, histamine and serotonin, negatively regulated β-cell differentiation. Reserpine or TBZ acted additively with dibutyryl adenosine 3',5'-cyclic AMP, a cell-permeable cAMP analog, to potentiate differentiation of embryonic stem (ES) cells into β cells that exhibited glucose-stimulated insulin secretion. When ES cell–derived β cells were transplanted into AKITA diabetic mice, the cells reversed hyperglycemia. Our protocol provides a basis for the understanding of β-cell differentiation and its application to a cost-effective production of functional β cells for cell therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Jonsson, J., Carlsson, L., Edlund, T. & Edlund, H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature 371, 606–609 (1994).
Gu, G., Dubauskaite, J. & Melton, D.A. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129, 2447–2457 (2002).
Gradwohl, G., Dierich, A., LeMeur, M. & Guillemot, F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc. Natl. Acad. Sci. USA 97, 1607–1611 (2000).
Puri, S. & Hebrok, M. Cellular plasticity within the pancreas—lessons learned from development. Dev. Cell 18, 342–356 (2010).
D′Amour, K.A. et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 23, 1534–1541 (2005).
Skoudy, A. et al. Transforming growth factor (TGF)β, fibroblast growth factor (FGF) and retinoid signalling pathways promote pancreatic exocrine gene expression in mouse embryonic stem cells. Biochem. J. 379, 749–756 (2004).
D′Amour, K.A. et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 24, 1392–1401 (2006).
Borowiak, M. et al. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell 4, 348–358 (2009).
Chen, S. et al. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat. Chem. Biol. 5, 258–265 (2009).
Shiraki, N. et al. Guided differentiation of embryonic stem cells into Pdx1-expressing regional-specific definitive endoderm. Stem Cells 26, 874–885 (2008).
Higuchi, Y. et al. Synthesized basement membranes direct the differentiation of mouse embryonic stem cells into pancreatic lineages. J. Cell Sci. 123, 2733–2742 (2010).
Wang, Y.M. et al. Knockout of the vesicular monoamine transporter 2 gene results in neonatal death and supersensitivity to cocaine and amphetamine. Neuron 19, 1285–1296 (1997).
Pothos, E.N. et al. Synaptic vesicle transporter expression regulates vesicle phenotype and quantal size. J. Neurosci. 20, 7297–7306 (2000).
Vergo, S., Johansen, J.L., Leist, M. & Lotharius, J. Vesicular monoamine transporter 2 regulates the sensitivity of rat dopaminergic neurons to disturbed cytosolic dopamine levels. Brain Res. 1185, 18–32 (2007).
Eiden, L.E. & Weihe, E. VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse. Ann. NY Acad. Sci. 1216, 86–98 (2011).
Erickson, J.D., Schafer, M.K., Bonner, T.I., Eiden, L.E. & Weihe, E. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc. Natl. Acad. Sci. USA 93, 5166–5171 (1996).
Anlauf, M. et al. Expression of the two isoforms of the vesicular monoamine transporter (VMAT1 and VMAT2) in the endocrine pancreas and pancreatic endocrine tumors. J. Histochem. Cytochem. 51, 1027–1040 (2003).
Simpson, N.R. et al. Visualizing pancreatic β-cell mass with [11C]DTBZ. Nucl. Med. Biol. 33, 855–864 (2006).
Saisho, Y. et al. Relationship between pancreatic vesicular monoamine transporter 2 (VMAT2) and insulin expression in human pancreas. J. Mol. Histol. 39, 543–551 (2008).
Grimsby, J., Chen, K., Wang, L.J., Lan, N.C. & Shih, J.C. Human monoamine oxidase A and B genes exhibit identical exon-intron organization. Proc. Natl. Acad. Sci. USA 88, 3637–3641 (1991).
Gu, G. et al. Global expression analysis of gene regulatory pathways during endocrine pancreatic development. Development 131, 165–179 (2004).
Kroeze, W.K., Sheffler, D.J. & Roth, B.L. G-protein-coupled receptors at a glance. J. Cell Sci. 116, 4867–4869 (2003).
Seino, S., Shibasaki, T. & Minami, K. Dynamics of insulin secretion and the clinical implications for obesity and diabetes. J. Clin. Invest. 121, 2118–2125 (2011).
Lo, K.W., Kan, H.M., Ashe, K.M. & Laurencin, C.T. The small molecule PKA-specific cyclic AMP analogue as an inducer of osteoblast-like cells differentiation and mineralization. J. Tissue Eng. Regen. Med. 6, 40–48 (2012).
Mei, F.C. et al. Differential signaling of cyclic AMP: opposing effects of exchange protein directly activated by cyclic AMP and cAMP-dependent protein kinase on protein kinase B activation. J. Biol. Chem. 277, 11497–11504 (2002).
Kelley, G.G. et al. Glucose-dependent potentiation of mouse islet insulin secretion by Epac activator 8-pCPT-2′-O-Me-cAMP-AM. Islets 1, 260–265 (2009).
Wang, J. et al. A mutation in the insulin 2 gene induces diabetes with severe pancreatic β-cell dysfunction in the Mody mouse. J. Clin. Invest. 103, 27–37 (1999).
Mochida, T. et al. Time-dependent changes in the plasma amino acid concentration in diabetes mellitus. Mol. Genet. Metab. 103, 406–409 (2011).
Hong, E.G. et al. Nonobese, insulin-deficient Ins2Akita mice develop type 2 diabetes phenotypes including insulin resistance and cardiac remodeling. Am. J. Physiol. Endocrinol. Metab. 293, E1687–E1696 (2007).
Schäfer, M.K. et al. Species-specific vesicular monoamine transporter 2 (VMAT2) expression in mammalian pancreatic β cells: implications for optimising radioligand-based human β cell mass (BCM) imaging in animal models. Diabetologia 56, 1047–1056 (2013).
Harris, P.E. et al. VMAT2 gene expression and function as it applies to imaging β-cell mass. J. Mol. Med. 86, 5–16 (2008).
Fülöp, A.K. et al. Hyperleptinemia, visceral adiposity, and decreased glucose tolerance in mice with a targeted disruption of the histidine decarboxylase gene. Endocrinology 144, 4306–4314 (2003).
Buchanan, T.A. & Xiang, A.H. Gestational diabetes mellitus. J. Clin. Invest. 115, 485–491 (2005).
Kim, H. et al. Serotonin regulates pancreatic β cell mass during pregnancy. Nat. Med. 16, 804–808 (2010).
Rubí, B. et al. Dopamine D2-like receptors are expressed in pancreatic β cells and mediate inhibition of insulin secretion. J. Biol. Chem. 280, 36824–36832 (2005).
Ericson, L.E., Hakanson, R. & Lundquist, I. Accumulation of dopamine in mouse pancreatic B-cells following injection of l-DOPA. Localization to secretory granules and inhibition of insulin secretion. Diabetologia 13, 117–124 (1977).
Zern, R.T., Bird, J.L. & Feldman, J.M. Effect of increased pancreatic islet norepinephrine, dopamine and serotonin concentration on insulin secretion in the golden hamster. Diabetologia 18, 341–346 (1980).
Seeman, P. et al. The dopaminergic stabilizer ASP2314/ACR16 selectively interacts with D2High receptors. Synapse 63, 930–934 (2009).
Braden, M.R., Parrish, J.C., Naylor, J.C. & Nichols, D.E. Molecular interaction of serotonin 5–HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists. Mol. Pharmacol. 70, 1956–1964 (2006).
Peakman, M.C. & Hill, S.J. Endogenous expression of histamine H1 receptors functionally coupled to phosphoinositide hydrolysis in C6 glioma cells: regulation by cyclic AMP. Br. J. Pharmacol. 113, 1554–1560 (1994).
El Mestikawy, S., Wallen-Mackenzie, A., Fortin, G.M., Descarries, L. & Trudeau, L.E. From glutamate co-release to vesicular synergy: vesicular glutamate transporters. Nat. Rev. Neurosci. 12, 204–216 (2011).
Yao, J., Erickson, J.D. & Hersh, L.B. Protein kinase A affects trafficking of the vesicular monoamine transporters in PC12 cells. Traffic 5, 1006–1016 (2004).
Xie, T., Chen, M. & Weinstein, L.S. Pancreas-specific Gsα deficiency has divergent effects on pancreatic α- and β-cell proliferation. J. Endocrinol. 206, 261–269 (2010).
Herrera, P.L. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development 127, 2317–2322 (2000).
Murtaugh, L.C. Pancreas and β-cell development: from the actual to the possible. Development 134, 427–438 (2007).
Collombat, P. et al. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently β cells. Cell 138, 449–462 (2009).
Thorel, F. et al. Conversion of adult pancreatic α-cells to β-cells after extreme β-cell loss. Nature 464, 1149–1154 (2010).
Xie, R. et al. Dynamic chromatin remodeling mediated by polycomb proteins orchestrates pancreatic differentiation of human embryonic stem cells. Cell Stem Cell 12, 224–237 (2013).
Kelly, O.G. et al. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat. Biotechnol. 29, 750–756 (2011).
Yasunami, Y. et al. Vα14 NK T cell-triggered IFN-γ production by Gr-1+CD11b+ cells mediates early graft loss of syngeneic transplanted islets. J. Exp. Med. 202, 913–918 (2005).
Acknowledgements
We thank members of the Gene Technology Center and Center for Animal Resources and Development at Kumamoto University for technical assistance. This work was supported by the Funding Program for Next Generation World-Leading Researchers (to S.K. (no. LS099) and M.U.); the Japan Society for the Promotion of Science, the Realization of Regenerative Medicine (to S.K. and M.U.); the Program for Leading Graduate Schools 'HIGO' (awarded to S.K.); Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan (no. 21390280 to S.K. and no. 22790653 to D. Sakano); and the Collaborative Research Program of Institute for Chemical Research, Kyoto University (grant no. 2010-44). The iCeMS is supported by World Premier International Research Center Initiative, MEXT, Japan.
Author information
Authors and Affiliations
Contributions
D. Sakano performed chemical screening, cellular and biochemical analyses; D. Sakano, N.S. and K.U. established the ES cell differentiation system; D. Sakano, K. Kikawa, M.K. and T.Y. performed transplantation assays; K.A. established the ES cell line; S.M., F.E. and N.N. helped maintain AKITA mice; D.M. and M.U. provided and analyzed the chemical library; O.A. and D. Stainier provided chemicals; K. Kume and S.K. provided technical advices, S.K. designed the experiments and wrote the paper. All of the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Figures 1–13 and Supplementary Tables 1 and 2. (PDF 2648 kb)
Rights and permissions
About this article
Cite this article
Sakano, D., Shiraki, N., Kikawa, K. et al. VMAT2 identified as a regulator of late-stage β-cell differentiation. Nat Chem Biol 10, 141–148 (2014). https://doi.org/10.1038/nchembio.1410
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1410
This article is cited by
-
Author contributions and allocation of authorship credit: testing the validity of different counting methods in the field of chemical biology
Scientometrics (2023)
-
Therapeutic potential of dopamine agonists in the treatment of type 2 diabetes mellitus
Environmental Science and Pollution Research (2022)
-
Recent progress in pancreatic islet cell therapy
Inflammation and Regeneration (2021)
-
A novel optical tracer for VMAT2 applied to live cell measurements of vesicle maturation in cultured human β-cells
Scientific Reports (2019)
-
Decreased VMAT2 in the pancreas of humans with type 2 diabetes mellitus measured in vivo by PET imaging
Diabetologia (2018)