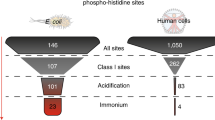
Abstract
Despite its importance in central metabolism and bacterial cell signaling, protein histidine phosphorylation has remained elusive with respect to its extent and functional roles in biological systems because of the lack of adequate research tools. We report the development of the first pan-phosphohistidine (pHis) antibody using a stable pHis mimetic as the hapten. This antibody was successfully used in ELISA, western blotting, dot blot assays and immunoprecipitation and in detection and identification of histidine-phosphorylated proteins from native cell lysates when coupled with MS analysis. We also observed that the amount of protein pHis in Escherichia coli lysates depends on carbon source and nitrogen availability in the growth medium. In particular, we found that the amount of pHis on phosphoenolpyruvate synthase (PpsA) is sensitive to nitrogen availability in vivo and that α-ketoglutarate inhibits phosphotransfer from phosphorylated PpsA to pyruvate. We expect this antibody to open opportunities for investigating other pHis proteins and their functions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Walsh, C.T. Posttranslational Modification of Proteins: Expanding Nature's Inventory Ch. 2 (Roberts and Company Publishers, Greenwood Village, 2006).
Grimsrud, P.A., Swaney, D.L., Wenger, C.D., Beauchene, N.A. & Coon, J.J. Phosphoproteomics for the masses. ACS Chem. Biol. 5, 105–119 (2010).
Kee, J.-M. & Muir, T.W. Chasing phosphohistidine, an elusive sibling in the phosphoamino acid family. ACS Chem. Biol. 7, 44–51 (2012).
Matthews, H.R. Protein kinases and phosphatases that act on histidine, lysine, or arginine residues in eukaryotic proteins: a possible regulator of the mitogen-activated protein kinase cascade. Pharmacol. Ther. 67, 323–350 (1995).
Attwood, P.V., Besant, P.G. & Piggott, M.J. Focus on phosphoaspartate and phosphoglutamate. Amino Acids 40, 1035–1051 (2011).
Boyer, P.D., Peter, J.B., Ebner, K.E., Deluca, M. & Hultquist, D.E. Identification of phosphohistidine in digests from a probable intermediate of oxidative phosphorylation. J. Biol. Chem. 237, PC3306–PC3308 (1962).
Attwood, P.V., Piggott, M.J., Zu, X.L. & Besant, P.G. Focus on phosphohistidine. Amino Acids 32, 145–156 (2007).
Rikova, K. et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 131, 1190–1203 (2007).
Olsen, J.V. et al. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 127, 635–648 (2006).
Carlson, H.K. et al. Use of a semisynthetic epitope to probe histidine kinase activity and regulation. Anal. Biochem. 397, 139–143 (2010).
Kee, J.-M., Villani, B., Carpenter, L.R. & Muir, T.W. Development of stable phosphohistidine analogues. J. Am. Chem. Soc. 132, 14327–14329 (2010).
McAllister, T.E., Nix, M.G. & Webb, M.E. Fmoc-chemistry of a stable phosphohistidine analogue. Chem. Commun. (Camb.) 47, 1297–1299 (2011).
Mukai, S. et al. Stable triazolylphosphonate analogues of phosphohistidine. Amino Acids 43, 857–874 (2012).
Hunter, T. Tyrosine phosphorylation: thirty years and counting. Curr. Opin. Cell Biol. 21, 140–146 (2009).
Rush, J. et al. Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nat. Biotechnol. 23, 94–101 (2005).
Frackelton, A.R., Posner, M., Kannan, B. & Mermelstein, F. Generation of monoclonal antibodies against phosphotyrosine and their use for affinity purification of phosphotyrosine-containing proteins. Methods Enzymol. 201, 79–92 (1991).
Duclos, B., Marcandier, S. & Cozzone, A.J. Chemical properties and separation of phosphoamino acids by thin-layer chromatography and or electrophoresis. Methods Enzymol. 201, 10–21 (1991).
Meadow, N.D., Fox, D.K. & Roseman, S. The bacterial phosphoenol-pyruvate: glycose phosphotransferase system. Annu. Rev. Biochem. 59, 497–542 (1990).
Waygood, E.B. & Steeves, T. Enzyme I of the phosphoenolpyruvate-sugar phosphotransferase system of Escherichia coli—purification to homogeneity and some properties. Can. J. Biochem. 58, 40–48 (1980).
Venditti, V., Ghirlando, R. & Clore, G.M. Structural basis for enzyme I inhibition by α-ketoglutarate. ACS Chem. Biol. published online, http://dx.doi.org/10.1021/cb400027q (2013).
Doucette, C.D., Schwab, D.J., Wingreen, N.S. & Rabinowitz, J.D. α-Ketoglutarate coordinates carbon and nitrogen utilization via enzyme I inhibition. Nat. Chem. Biol. 7, 894–901 (2011).
Chulavatnatol, M. & Atkinson, D.E. Phosphoenolpyruvate synthetase from Escherichia coli. Effects of adenylate energy charge and modifier concentrations. J. Biol. Chem. 248, 2712–2715 (1973).
Yuan, J. et al. Metabolomics-driven quantitative analysis of ammonia assimilation in E. coli. Mol. Syst. Biol. 5, 302 (2009).
Lasker, M. et al. Protein histidine phosphorylation: increased stability of thiophosphohistidine. Protein Sci. 8, 2177–2185 (1999).
Schenkels, C., Erni, B. & Reymond, J. Phosphofurylalanine, a stable analog of phosphohistidine. Bioorg. Med. Chem. Lett. 9, 1443–1446 (1999).
Klumpp, S. & Krieglstein, J. Reversible phosphorylation of histidine residues in proteins from vertebrates. Sci. Signal. 2, pe13 (2009).
Frackelton, A.R., Ross, A.H. & Eisen, H.N. Characterization and use of monoclonal antibodies for isolation of phosphotyrosyl proteins from retrovirus-transformed cells and growth factor–stimulated cells. Mol. Cell Biol. 3, 1343–1352 (1983).
Paul, R. et al. Allosteric regulation of histidine kinases by their cognate response regulator determines cell fate. Cell 133, 452–461 (2008).
Stock, A.M., Robinson, V.L. & Goudreau, P.N. Two-component signal transduction. Annu. Rev. Biochem. 69, 183–215 (2000).
Patnaik, R. & Liao, J.C. Engineering of Escherichia coli central metabolism for aromatic metabolite production with near theoretical yield. Appl. Environ. Microbiol. 60, 3903–3908 (1994).
Acknowledgements
We would like to acknowledge S. Darst and M. Bick (The Rockefeller University) for providing KinB and J. Rabinowitz and M. Reaves (Princeton University) for providing NCM 3722 cells. We thank J. Rabinowitz, M. Reaves and B. Wang for stimulating discussions and S. Kyin and H. Shwe for their assistance in MS. This work was funded by the US National Institutes of Health (5R01GM095880). J.-M.K. and R.C.O. were supported by a postdoctoral fellowship from the Damon Runyon Cancer Research Foundation (DRG-2005-09) and a National Institutes of Health Research Service Award (1F32CA167901), respectively.
Author information
Authors and Affiliations
Contributions
J.-M.K., R.C.O. and T.W.M. designed the experiments. J.-M.K., R.C.O. and D.H.P. performed the experiments. J.-M.K. and R.C.O. prepared new reagents. Experimental results were analyzed and the manuscript was written by J.-M.K., R.C.O., D.H.P. and T.W.M.
Corresponding author
Ethics declarations
Competing interests
J.-M.K. and T.W.M. are co-inventors of a patent application on stable phosphohistidine analogs.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Notes 1–9 (PDF 43558 kb)
Rights and permissions
About this article
Cite this article
Kee, JM., Oslund, R., Perlman, D. et al. A pan-specific antibody for direct detection of protein histidine phosphorylation. Nat Chem Biol 9, 416–421 (2013). https://doi.org/10.1038/nchembio.1259
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1259
This article is cited by
-
Phosphohistidine signaling promotes FAK-RB1 interaction and growth factor-independent proliferation of esophageal squamous cell carcinoma
Oncogene (2023)
-
A parallel glycolysis provides a selective advantage through rapid growth acceleration
Nature Chemical Biology (2023)
-
Histidine phosphorylation in human cells; a needle or phantom in the haystack?
Nature Methods (2022)
-
Bis(zinc(II)-dipicolylamine)-functionalized sub-2 μm core-shell microspheres for the analysis of N-phosphoproteome
Nature Communications (2020)
-
Protein post-translational modifications in bacteria
Nature Reviews Microbiology (2019)