Abstract
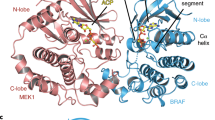
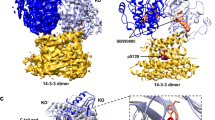
RAF kinases have a prominent role in cancer. Their mode of activation is complex but critically requires dimerization of their kinase domains. Unexpectedly, several ATP-competitive RAF inhibitors were recently found to promote dimerization and transactivation of RAF kinases in a RAS-dependent manner and, as a result, undesirably stimulate RAS/ERK pathway–mediated cell growth. The mechanism by which these inhibitors induce RAF kinase domain dimerization remains unclear. Here we describe bioluminescence resonance energy transfer–based biosensors for the extended RAF family that enable the detection of RAF dimerization in living cells. Notably, we demonstrate the utility of these tools for profiling kinase inhibitors that selectively modulate RAF dimerization and for probing structural determinants of RAF dimerization in vivo. Our findings, which seem generalizable to other kinase families allosterically regulated by kinase domain dimerization, suggest a model whereby ATP-competitive inhibitors mediate RAF dimerization by stabilizing a rigid closed conformation of the kinase domain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Roberts, P.J. & Der, C.J. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 26, 3291–3310 (2007).
Wellbrock, C., Karasarides, M. & Marais, R. The RAF proteins take centre stage. Nat. Rev. Mol. Cell Biol. 5, 875–885 (2004).
Dhomen, N. & Marais, R. New insight into BRAF mutations in cancer. Curr. Opin. Genet. Dev. 17, 31–39 (2007).
Schubbert, S., Shannon, K. & Bollag, G. Hyperactive Ras in developmental disorders and cancer. Nat. Rev. Cancer 7, 295–308 (2007).
Clapéron, A. & Therrien, M. KSR and CNK: two scaffolds regulating RAS-mediated RAF activation. Oncogene 26, 3143–3158 (2007).
Garnett, M.J., Rana, S., Paterson, H., Barford, D. & Marais, R. Wild-type and mutant B-RAF activate C-RAF through distinct mechanisms involving heterodimerization. Mol. Cell 20, 963–969 (2005).
Rajakulendran, T., Sahmi, M., Lefrancois, M., Sicheri, F. & Therrien, M. A dimerization-dependent mechanism drives RAF catalytic activation. Nature 461, 542–545 (2009).
Rushworth, L.K., Hindley, A.D., O'Neill, E. & Kolch, W. Regulation and role of Raf-1/B-Raf heterodimerization. Mol. Cell Biol. 26, 2262–2272 (2006).
Weber, C.K., Slupsky, J.R., Kalmes, H.A. & Rapp, U.R. Active Ras induces heterodimerization of cRaf and BRaf. Cancer Res. 61, 3595–3598 (2001).
Halilovic, E. & Solit, D.B. Therapeutic strategies for inhibiting oncogenic BRAF signaling. Curr. Opin. Pharmacol. 8, 419–426 (2008).
Bollag, G. et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 467, 596–599 (2010).
Joseph, E.W. et al. The RAF inhibitor PLX4032 inhibits ERK signaling and tumor cell proliferation in a V600E BRAF-selective manner. Proc. Natl. Acad. Sci. USA 107, 14903–14908 (2010).
Hatzivassiliou, G. et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature 464, 431–435 (2010).
Heidorn, S.J. et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell 140, 209–221 (2010).
Poulikakos, P.I., Zhang, C., Bollag, G., Shokat, K.M. & Rosen, N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 464, 427–430 (2010).
Poulikakos, P.I. & Rosen, N. Mutant BRAF melanomas—dependence and resistance. Cancer Cell 19, 11–15 (2011).
Poulikakos, P.I. et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature 480, 387–390 (2011).
Solit, D.B. & Rosen, N. Resistance to BRAF inhibition in melanomas. N. Engl. J. Med. 364, 772–774 (2011).
Bacart, J., Corbel, C., Jockers, R., Bach, S. & Couturier, C. The BRET technology and its application to screening assays. Biotechnol. J. 3, 311–324 (2008).
Breton, B. et al. Multiplexing of multicolor bioluminescence resonance energy transfer. Biophys. J. 99, 4037–4046 (2010).
Kocan, M., See, H.B., Seeber, R.M., Eidne, K.A. & Pfleger, K.D. Demonstration of improvements to the bioluminescence resonance energy transfer (BRET) technology for the monitoring of G protein–coupled receptors in live cells. J. Biomol. Screen. 13, 888–898 (2008).
James, J.R., Oliveira, M.I., Carmo, A.M., Iaboni, A. & Davis, S.J. A rigorous experimental framework for detecting protein oligomerization using bioluminescence resonance energy transfer. Nat. Methods 3, 1001–1006 (2006).
Röring, M. et al. Distinct requirement for an intact dimer interface in wild-type, V600E and kinase-dead B-Raf signalling. EMBO J. 31, 2629–2647 (2012).
Ritt, D.A., Monson, D.M., Specht, S.I. & Morrison, D.K. Impact of feedback phosphorylation and Raf heterodimerization on normal and mutant B-Raf signaling. Mol. Cell Biol. 30, 806–819 (2010).
Dar, A.C. & Shokat, K.M. The evolution of protein kinase inhibitors from antagonists to agonists of cellular signaling. Annu. Rev. Biochem. 80, 769–795 (2011).
Whittaker, S. et al. Gatekeeper mutations mediate resistance to BRAF-targeted therapies. Sci. Transl. Med. 2, 35ra41 (2010).
Zhang, J.H., Chung, T.D. & Oldenburg, K.R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 4, 67–73 (1999).
Tsai, J. et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc. Natl. Acad. Sci. USA 105, 3041–3046 (2008).
Wan, P.T. et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 116, 855–867 (2004).
Lee, J.C. et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372, 739–746 (1994).
Hall-Jackson, C.A., Goedert, M., Hedge, P. & Cohen, P. Effect of SB 203580 on the activity of c-Raf in vitro and in vivo. Oncogene 18, 2047–2054 (1999).
Kalmes, A., Deou, J., Clowes, A.W. & Daum, G. Raf-1 is activated by the p38 mitogen-activated protein kinase inhibitor, SB203580. FEBS Lett. 444, 71–74 (1999).
McKay, M.M., Ritt, D.A. & Morrison, D.K. RAF Inhibitor-Induced KSR1/B-RAF binding and its effects on ERK cascade signaling. Curr. Biol. 21, 563–568 (2011).
King, A.J. et al. Demonstration of a genetic therapeutic index for tumors expressing oncogenic BRAF by the kinase inhibitor SB-590885. Cancer Res. 66, 11100–11105 (2006).
Wang, Z. et al. Structural basis of inhibitor selectivity in MAP kinases. Structure 6, 1117–1128 (1998).
Anastassiadis, T., Deacon, S.W., Devarajan, K., Ma, H. & Peterson, J.R. Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat. Biotechnol. 29, 1039–1045 (2011).
Packer, L.M. et al. Nilotinib and MEK inhibitors induce synthetic lethality through paradoxical activation of RAF in drug-resistant chronic myeloid leukemia. Cancer Cell 20, 715–727 (2011).
Barrios-Rodiles, M. et al. High-throughput mapping of a dynamic signaling network in mammalian cells. Science 307, 1621–1625 (2005).
Jeffrey, P.D. et al. Mechanism of CDK activation revealed by the structure of a cyclinA–CDK2 complex. Nature 376, 313–320 (1995).
Sicheri, F. & Kuriyan, J. Structures of Src-family tyrosine kinases. Curr. Opin. Struct. Biol. 7, 777–785 (1997).
Kornev, A.P., Haste, N.M., Taylor, S.S. & Eyck, L.F. Surface comparison of active and inactive protein kinases identifies a conserved activation mechanism. Proc. Natl. Acad. Sci. USA 103, 17783–17788 (2006).
Kornev, A.P. & Taylor, S.S. Defining the conserved internal architecture of a protein kinase. Biochim. Biophys. Acta 1804, 440–444 (2010).
Bukhtiyarova, M., Karpusas, M., Northrop, K., Namboodiri, H.V. & Springman, E.B. Mutagenesis of p38α MAP kinase establishes key roles of Phe169 in function and structural dynamics and reveals a novel DFG-OUT state. Biochemistry 46, 5687–5696 (2007).
Axten, J.M. et al. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-p yrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase r (PKR)-like endoplasmic reticulum kinase (PERK). J. Med. Chem. 55, 7193–7207 (2012).
Dey, M. et al. Mechanistic link between PKR dimerization, autophosphorylation, and eIF2α substrate recognition. Cell 122, 901–913 (2005).
Taylor, S.S., Haste, N.M. & Ghosh, G. PKR and eIF2α: integration of kinase dimerization, activation, and substrate docking. Cell 122, 823–825 (2005).
Korennykh, A.V. et al. The unfolded protein response signals through high-order assembly of Ire1. Nature 457, 687–693 (2009).
Sen, B. et al. Kinase-impaired BRAF mutations in lung cancer confer sensitivity to dasatinib. Sci. Transl. Med. 4, 136ra170 (2012).
Dar, A.C., Dever, T.E. & Sicheri, F. Higher-order substrate recognition of eIF2α by the RNA-dependent protein kinase PKR. Cell 122, 887–900 (2005).
Boussif, O. et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. USA 92, 7297–7301 (1995).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Ciruela, F. Fluorescence-based methods in the study of protein-protein interactions in living cells. Curr. Opin. Biotechnol. 19, 338–343 (2008).
Acknowledgements
We thank B. Breton and M. Audet for advice with the BRET2 system, D. Uehling and co-workers at the Ontario Institute for Cancer Research for GSK2606414, and the Institut de recherche en immunologie et en cancérologie (IRIC) high-throughput screening platform. IRIC is supported by the Canadian Center of Excellence in Commercialization and Research, the Canada Foundation for Innovation and by the Fonds de Recherche du Québec en Santé. H.L. is a recipient of Cancer Research Society and Canadian Institutes for Health Research (CIHR) Banting postdoctoral fellowships. N.T. is a recipient of the CIHR Canadian Graduate Scholarship. M.B., F.S. and M.T. hold Canada Research Chairs. This work was supported by operating funds from the Canadian Cancer Society to M.T. (018046) and from the CIHR to M.T. (MOP119443) and F.S. (MOP36399).
Author information
Authors and Affiliations
Contributions
H.L., N.T., F.S. and M.T. designed the experiments and wrote the manuscript. M.B. contributed to the theoretical framework surrounding BRET assay development and participated in the analysis of the BRET data and in the revision of the manuscript. H.L., with assistance from G.G., A.P., S.G. and J.D., conducted cell-based BRET analyses, the high-throughput chemical screen and TR-FRET in vitro binding assays. N.T., with assistance from D.Y.L.M., J.J.L. and H.L., performed AUC and X-ray structure analyses.
Corresponding authors
Ethics declarations
Competing interests
H.L., F.S. and M.T. have filed a patent covering the BRET-based method described in this study for detecting RAF dimerization.
Supplementary information
Supplementary Text and Figures
Supplementary Results (PDF 73424 kb)
Supplementary Data Set 1
Supplementary Data Set 1 (XLSX 56 kb)
Supplementary Note 1
Supplementary Note 1 (PDF 561 kb)
Rights and permissions
About this article
Cite this article
Lavoie, H., Thevakumaran, N., Gavory, G. et al. Inhibitors that stabilize a closed RAF kinase domain conformation induce dimerization. Nat Chem Biol 9, 428–436 (2013). https://doi.org/10.1038/nchembio.1257
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1257
This article is cited by
-
Analysis of RAS and drug induced homo- and heterodimerization of RAF and KSR1 proteins in living cells using split Nanoluc luciferase
Cell Communication and Signaling (2023)
-
Multiomic analysis of papillary thyroid cancers identifies BAIAP2L1-BRAF fusion and requirement of TRIM25, PDE5A and PKCδ for tumorigenesis
Molecular Cancer (2022)
-
GCN2 kinase activation by ATP-competitive kinase inhibitors
Nature Chemical Biology (2022)
-
Deacylation-aided C–H alkylative annulation through C–C cleavage of unstrained ketones
Nature Catalysis (2021)
-
Precision oncology in metastatic colorectal cancer — from biology to medicine
Nature Reviews Clinical Oncology (2021)