Abstract
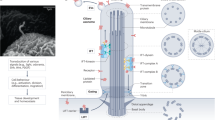
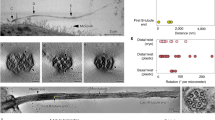
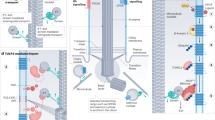
Primary cilia function as specialized compartments for signal transduction. The stereotyped structure and signaling function of cilia inextricably depend on the selective segregation of molecules in cilia. However, the fundamental principles governing the access of soluble proteins to primary cilia remain unresolved. We developed a methodology termed 'chemically inducible diffusion trap at cilia' to visualize the diffusion process of a series of fluorescent proteins ranging in size from 3.2 nm to 7.9 nm into primary cilia. We found that the interior of the cilium was accessible to proteins as large as 7.9 nm. The kinetics of ciliary accumulation of this panel of proteins was exponentially limited by their Stokes radii. Quantitative modeling suggests that the diffusion barrier operates as a molecular sieve at the base of cilia. Our study presents a set of powerful, generally applicable tools for the quantitative monitoring of ciliary protein diffusion under both physiological and pathological conditions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Singla, V. & Reiter, J.F. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science 313, 629–633 (2006).
Veland, I.R., Awan, A., Pedersen, L.B., Yoder, B.K. & Christensen, S.T. Primary cilia and signaling pathways in mammalian development, health and disease. Nephron Physiol. 111, 39–53 (2009).
Satir, P. & Christensen, S.T. Overview of structure and function of mammalian cilia. Annu. Rev. Physiol. 69, 377–400 (2007).
Nachury, M.V., Seeley, E.S. & Jin, H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu. Rev. Cell Dev. Biol. 26, 59–87 (2010).
Chih, B. et al. A ciliopathy complex at the transition zone protects the cilia as a privileged membrane domain. Nat. Cell Biol. 14, 61–72 (2012).
Francis, S.S., Sfakianos, J., Lo, B. & Mellman, I. A hierarchy of signals regulates entry of membrane proteins into the ciliary membrane domain in epithelial cells. J. Cell Biol. 193, 219–233 (2011).
Hu, Q. et al. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science 329, 436–439 (2010).
Calvert, P.D., Strissel, K.J., Schiesser, W.E., Pugh, E.N. Jr. & Arshavsky, V.Y. Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends Cell Biol. 16, 560–568 (2006).
Nair, K.S. et al. Light-dependent redistribution of arrestin in vertebrate rods is an energy-independent process governed by protein-protein interactions. Neuron 46, 555–567 (2005).
Najafi, M., Maza, N.A. & Calvert, P.D. Steric volume exclusion sets soluble protein concentrations in photoreceptor sensory cilia. Proc. Natl. Acad. Sci. USA 109, 203–208 (2012).
Kee, H.L. et al. A size-exclusion permeability barrier and nucleoporins characterize a ciliary pore complex that regulates transport into cilia. Nat. Cell Biol. 14, 431–437 (2012).
DeRose, R., Miyamoto, T. & Inoue, T. Manipulating signaling at will: chemically-inducible dimerization (CID) techniques resolve problems in cell biology. Pflugers Arch. 465, 409–417 (2013).
Berbari, N.F., Johnson, A.D., Lewis, J.S., Askwith, C.C. & Mykytyn, K. Identification of ciliary localization sequences within the third intracellular loop of G protein–coupled receptors. Mol. Biol. Cell 19, 1540–1547 (2008).
Komatsu, T. et al. Organelle-specific, rapid induction of molecular activities and membrane tethering. Nat. Methods 7, 206–208 (2010).
Miyamoto, T. et al. Rapid and orthogonal logic gating with a gibberellin-induced dimerization system. Nat. Chem. Biol. 8, 465–470 (2012).
Celada, F. & Zabin, I. A dimer-dimer binding region in β-galactosidase. Biochemistry 18, 404–406 (1979).
Matthews, B.W. The structure of E. coli β-galactosidase. C. R. Biol. 328, 549–556 (2005).
Seksek, O., Biwersi, J. & Verkman, A.S. Translational diffusion of macromolecule-sized solutes in cytoplasm and nucleus. J. Cell Biol. 138, 131–142 (1997).
Kärger, J., Chmelik, C., Heinke, L. & Valiullin, R. A new view of diffusion in nanoporous materials. Chemie Ingenieur Technik 82, 779–804 (2010).
Czlapinski, J.L. et al. Conditional glycosylation in eukaryotic cells using a biocompatible chemical inducer of dimerization. J. Am. Chem. Soc. 130, 13186–13187 (2008).
Narita, K., Kawate, T., Kakinuma, N. & Takeda, S. Multiple primary cilia modulate the fluid transcytosis in choroid plexus epithelium. Traffic 11, 287–301 (2010).
Okada, Y., Takeda, S., Tanaka, Y., Izpisua Belmonte, J.C. & Hirokawa, N. Mechanism of nodal flow: a conserved symmetry breaking event in left-right axis determination. Cell 121, 633–644 (2005).
Yoshimura, K., Kawate, T. & Takeda, S. Signaling through the primary cilium affects glial cell survival under a stressed environment. Glia 59, 333–344 (2011).
Dauty, E. & Verkman, A.S. Molecular crowding reduces to a similar extent the diffusion of small solutes and macromolecules: measurement by fluorescence correlation spectroscopy. J. Mol. Recognit. 17, 441–447 (2004).
Mohr, D., Frey, S., Fischer, T., Guttler, T. & Gorlich, D. Characterisation of the passive permeability barrier of nuclear pore complexes. EMBO J. 28, 2541–2553 (2009).
Chen, E.I., Hewel, J., Felding-Habermann, B. & Yates, J.R. 3rd. Large scale protein profiling by combination of protein fractionation and multidimensional protein identification technology (MudPIT). Mol. Cell Proteomics 5, 53–56 (2006).
D'Angelo, M.A. & Hetzer, M.W. Structure, dynamics and function of nuclear pore complexes. Trends Cell Biol. 18, 456–466 (2008).
Nimchinsky, E.A., Sabatini, B.L. & Svoboda, K. Structure and function of dendritic spines. Annu. Rev. Physiol. 64, 313–353 (2002).
Tada, T. et al. Role of Septin cytoskeleton in spine morphogenesis and dendrite development in neurons. Curr. Biol. 17, 1752–1758 (2007).
Sahimi, M. & Jue, V.L. Diffusion of large molecules in porous media. Phys. Rev. Lett. 62, 629–632 (1989).
Jensen, C.G. et al. Ultrastructural, tomographic and confocal imaging of the chondrocyte primary cilium in situ. Cell Biol. Int. 28, 101–110 (2004).
Graser, S. et al. Cep164, a novel centriole appendage protein required for primary cilium formation. J. Cell Biol. 179, 321–330 (2007).
Hildebrandt, F. & Zhou, W. Nephronophthisis-associated ciliopathies. J. Am. Soc. Nephrol. 18, 1855–1871 (2007).
Nachury, M.V. et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129, 1201–1213 (2007).
Salomon, R., Saunier, S. & Niaudet, P. Nephronophthisis. Pediatr. Nephrol. 24, 2333–2344 (2009).
Szymanska, K. & Johnson, C.A. The transition zone: an essential functional compartment of cilia. Cilia. 1, 10 (2012).
von Schnakenburg, C., Fliegauf, M. & Omran, H. Nephrocystin and ciliary defects not only in the kidney? Pediatr. Nephrol. 22, 765–769 (2007).
Inoue, T., Heo, W.D., Grimley, J.S., Wandless, T.J. & Meyer, T. An inducible translocation strategy to rapidly activate and inhibit small GTPase signaling pathways. Nat. Methods 2, 415–418 (2005).
Laurent, T.C. & Killander, J. A theory of gel filtration and its experimental verification. J. Chromatogr. A 14, 317–330 (1964).
Fukatsu, K. et al. Lateral diffusion of inositol 1,4,5-trisphosphate receptor type 1 is regulated by actin filaments and 4.1N in neuronal dendrites. J. Biol. Chem. 279, 48976–48982 (2004).
Acknowledgements
We thank A. Seki (Stanford University) for the 5HT6 construct, S. Takeda (University of Yamanashi) for the GFP-IFT88 construct and M. Wolfgang (Johns Hopkins University) for β-Gal and luciferase constructs. We also thank D.N.R., T.K., H.I. and S.T. for helpful discussions. This study was supported in part by the US National Institutes of Health (the Baltimore Polycystic Kidney Disease Research and Clinical Core Center provided pilot funds GM092930 and P30 DK090868 to Takanari Inoue and R00CA129174 and R21NS074091 to R.R.) and other grants (Grant-in-Aid for Challenging Exploratory Research 23650197 to H.N. and Pew Foundation to R.R.).
Author information
Authors and Affiliations
Contributions
Y.-C.L., S.C.P. and Takanari Inoue generated DNA constructs, and Y.-C.L., S.C.P. and J.J. performed cell biology experiments. B.L. analyzed data with R.R., P.N., A.L. and Takanari Inoue, and P.N. performed biochemical experiments with R.R. H.N. performed FRAP experiments with Takafumi Inoue, Y.-C.L., B.L. and R.R., and Takanari Inoue wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results (PDF 3860 kb)
Supplementary Video 1
Supplementary Movie 1 (AVI 3834 kb)
Rights and permissions
About this article
Cite this article
Lin, YC., Niewiadomski, P., Lin, B. et al. Chemically inducible diffusion trap at cilia reveals molecular sieve–like barrier. Nat Chem Biol 9, 437–443 (2013). https://doi.org/10.1038/nchembio.1252
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1252
This article is cited by
-
Transport and barrier mechanisms that regulate ciliary compartmentalization and ciliopathies
Nature Reviews Nephrology (2024)
-
A stress-induced cilium-to-PML-NB route drives senescence initiation
Nature Communications (2023)
-
Appearing and disappearing acts of cilia
Journal of Biosciences (2023)
-
PIP2 determines length and stability of primary cilia by balancing membrane turnovers
Communications Biology (2022)
-
Molecular exclusion limits for diffusion across a porous capsid
Nature Communications (2021)