Abstract
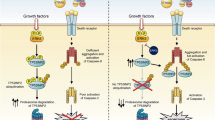
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) activates apoptosis through the death receptors DR4 and DR5. Because of its superior safety profile and high tumor specificity compared to other TNF family members, recombinant soluble TRAIL and agonistic antibodies against its receptors are actively being developed for clinical cancer therapy. Here, we describe the identification and characterization of the small molecules that directly target DR5 to initiate apoptosis in human cancer cells. The activity was initially discovered through a high-throughput chemical screen for compounds that promote cell death in synergy with a small-molecule mimetic of Smac, the antagonist for inhibitor of apoptosis protein. Structure-activity relationship studies yielded a more potent analog called bioymifi, which can act as a single agent to induce DR5 clustering and aggregation, leading to apoptosis. Thus, this study identified potential lead compounds for the development of small-molecule TRAIL mimics targeting DR5 for cancer therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Green, D.R. Apoptotic pathways: ten minutes to dead. Cell 121, 671–674 (2005).
Jiang, X. & Wang, X. Cytochrome c–mediated apoptosis. Annu. Rev. Biochem. 73, 87–106 (2004).
Ashkenazi, A. & Dixit, V.M. Death receptors: signaling and modulation. Science 281, 1305–1308 (1998).
Nagata, S. Apoptosis by death factor. Cell 88, 355–365 (1997).
Wilson, N.S., Dixit, V. & Ashkenazi, A. Death receptor signal transducers: nodes of coordination in immune signaling networks. Nat. Immunol. 10, 348–355 (2009).
Peter, M.E. & Krammer, P.H. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 10, 26–35 (2003).
Sprick, M.R. et al. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity 12, 599–609 (2000).
Kischkel, F.C. et al. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity 12, 611–620 (2000).
Varfolomeev, E. et al. Molecular determinants of kinase pathway activation by Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand. J. Biol. Chem. 280, 40599–40608 (2005).
Crook, N.E., Clem, R.J. & Miller, L.K. An apoptosis-inhibiting baculovirus gene with a zinc finger–like motif. J. Virol. 67, 2168–2174 (1993).
Deveraux, Q.L. et al. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 17, 2215–2223 (1998).
Roy, N., Deveraux, Q.L., Takahashi, R., Salvesen, G.S. & Reed, J.C. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 16, 6914–6925 (1997).
Li, L. et al. A small molecule Smac mimic potentiates TRAIL- and TNFα-mediated cell death. Science 305, 1471–1474 (2004).
Wang, L., Du, F. & Wang, X. TNF-α induces two distinct caspase-8 activation pathways. Cell 133, 693–703 (2008).
Petersen, S.L. et al. Autocrine TNFα signaling renders human cancer cells susceptible to Smac-mimetic–induced apoptosis. Cancer Cell 12, 445–456 (2007).
Hanahan, D. & Weinberg, R.A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Danial, N.N. & Korsmeyer, S.J. Cell death: critical control points. Cell 116, 205–219 (2004).
Vince, J.E. et al. IAP antagonists target cIAP1 to induce TNFα-dependent apoptosis. Cell 131, 682–693 (2007).
Wiezorek, J., Holland, P. & Graves, J. Death receptor agonists as a targeted therapy for cancer. Clin. Cancer Res. 16, 1701–1708 (2010).
Ashkenazi, A., Holland, P. & Eckhardt, S.G. Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL). J. Clin. Oncol. 26, 3621–3630 (2008).
Feoktistova, M. et al. cIAPs block ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol. Cell 43, 449–463 (2011).
Alberti, S., Halfmann, R., King, O., Kapila, A. & Lindquist, S. A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell 137, 146–158 (2009).
Hou, F. et al. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell 146, 448–461 (2011).
Jin, Z. et al. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell 137, 721–735 (2009).
Siegel, R.M. et al. SPOTS: signaling protein oligomeric transduction structures are early mediators of death receptor-induced apoptosis at the plasma membrane. J. Cell Biol. 167, 735–744 (2004).
Wagner, K.W. et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat. Med. 13, 1070–1077 (2007).
Fulda, S., Wick, W., Weller, M. & Debatin, K.M. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug–induced apoptosis and induce regression of malignant glioma in vivo. Nat. Med. 8, 808–815 (2002).
Chen, G. & Goeddel, D.V. TNF-R1 signaling: a beautiful pathway. Science 296, 1634–1635 (2002).
Acknowledgements
We thank M. Zhao for NMR and HPLC/MS analysis; S.L. Petersen for reagents and discussions; L. Zhao for the toxicological experiments; S.L. McKnight for helpful suggestions; M. Roth for proposing the chemical library screen; D. Frantz, J. Ready and A. Wang for synthesis of initial hits; Y. Liu, Z. Zhang, Q. Liu and L. Shang for comments of the manuscript; S.-C. Tso and T. Scheuermann for detecting the interaction between DR5 and bioymifi; and K. Phelps and A. Budge for the cell imaging. This work is supported by a program project grant from the US National Cancer Institute (2P01 CA095471-06 to Xiaodong Wang and G.W.) and the National Natural Science Foundation of China (21072150 and 21222209 to X.L.).
Author information
Authors and Affiliations
Contributions
Xiaodong Wang, X.L. and G.W. designed the study; G.W., H.Y., N.W., D.L.H., R.H., L.L. and L.W. performed and analyzed the biological experiments; Xiaoming Wang, J.N., P.H. and X.L. performed all of the chemical syntheses; S.C. performed the MS analysis; S.W. implemented the chemical library screen; and Xiaodong Wang, X.L. and G.W. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results (PDF 644 kb)
Supplementary Note 1
Supplementary Note 1 – general information (PDF 10516 kb)
Rights and permissions
About this article
Cite this article
Wang, G., Wang, X., Yu, H. et al. Small-molecule activation of the TRAIL receptor DR5 in human cancer cells. Nat Chem Biol 9, 84–89 (2013). https://doi.org/10.1038/nchembio.1153
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1153
This article is cited by
-
Death receptor 5 is required for intestinal stem cell activity during intestinal epithelial renewal at homoeostasis
Cell Death & Disease (2024)
-
Bioymifi, a novel mimetic of TNF-related apoptosis-induced ligand (TRAIL), stimulates eryptosis
Medical Oncology (2021)
-
Quinacrine causes apoptosis in human cancer cell lines through caspase-mediated pathway and regulation of small-GTPase
Journal of Biosciences (2020)
-
Thermodynamic properties of some isomeric 5-(nitrophenyl)-furyl-2 derivatives
BMC Chemistry (2019)
-
The VP3 protein of duck hepatitis A virus mediates host cell adsorption and apoptosis
Scientific Reports (2019)