Abstract
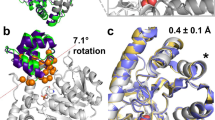
Engineering functional protein scaffolds capable of carrying out chemical catalysis is a major challenge in enzyme design. Starting from a noncatalytic protein scaffold, we recently generated a new RNA ligase by in vitro directed evolution. This artificial enzyme lost its original fold and adopted an entirely new structure with substantially enhanced conformational dynamics, demonstrating that a primordial fold with suitable flexibility is sufficient to carry out enzymatic function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Chothia, C. Nature 357, 543–544 (1992).
Murzin, A.G., Brenner, S.E., Hubbard, T. & Chothia, C. J. Mol. Biol. 247, 536–540 (1995).
Ohno, S. Evolution by Gene Duplication (Springer-Verlag, New York, 1971).
Chothia, C., Gough, J., Vogel, C. & Teichmann, S.A. Science 300, 1701–1703 (2003).
James, L.C. & Tawfik, D.S. Trends Biochem. Sci. 28, 361–368 (2003).
Tokuriki, N. & Tawfik, D.S. Science 324, 203–207 (2009).
Cordes, M.H.J., Walsh, N.P., McKnight, C.J. & Sauer, R.T. Science 284, 325–328 (1999).
Kaplan, J. & DeGrado, W.F. Proc. Natl. Acad. Sci. USA 101, 11566–11570 (2004).
Tuinstra, R.L. et al. Proc. Natl. Acad. Sci. USA 105, 5057–5062 (2008).
Bryan, P.N. & Orban, J. Curr. Opin. Struct. Biol. 20, 482–488 (2010).
Smith, B.A. & Hecht, M.H. Curr. Opin. Chem. Biol. 15, 421–426 (2011).
Keefe, A.D. & Szostak, J.W. Nature 410, 715–718 (2001).
Mansy, S.S. et al. J. Mol. Biol. 371, 501–513 (2007).
Seelig, B. & Szostak, J.W. Nature 448, 828–831 (2007).
Seelig, B. Nat. Protoc. 6, 540–552 (2011).
Holmbeck, S.M.A. et al. J. Mol. Biol. 281, 271–284 (1998).
Cho, G.S. & Szostak, J.W. Chem. Biol. 13, 139–147 (2006).
Zhao, Q. et al. J. Mol. Biol. 296, 509–520 (2000).
Maret, W. & Li, Y. Chem. Rev. 109, 4682–4707 (2009).
van Tilborg, P.J. et al. Biochemistry 39, 8747–8757 (2000).
Yang, W., Lee, J.Y. & Nowotny, M. Mol. Cell 22, 5–13 (2006).
Bhabha, G. et al. Science 332, 234–238 (2011).
Baldwin, A.J. & Kay, L.E. Nat. Chem. Biol. 5, 808–814 (2009).
Henzler-Wildman, K. & Kern, D. Nature 450, 964–972 (2007).
Golynskiy, M.V. & Seelig, B. Trends Biotechnol. 28, 340–345 (2010).
Grzesiek, S. & Bax, A. J. Magn. Reson. 96, 432–440 (1992).
Muhandiram, D.R. & Kay, L.E. J. Magn. Reson. B. 103, 203–216 (1994).
Wittekind, M. & Mueller, L. J. Magn. Reson. B. 101, 201–205 (1993).
Eghbalnia, H.R., Bahrami, A., Tonelli, M., Hallenga, K. & Markley, J.L. J. Am. Chem. Soc. 127, 12528–12536 (2005).
Grzesiek, S., Anglister, J. & Bax, A. J. Magn. Reson. B. 101, 114–119 (1993).
Wuthrich, K. NMR of Proteins and Nucleic Acids (John Wiley and Sons, New York, 1986).
Wishart, D.S., Sykes, B.D. & Richards, F.M. J. Mol. Biol. 222, 311–333 (1991).
Vuister, G.W. & Bax, A. J. Am. Chem. Soc. 115, 7772–7777 (1993).
Lee, D., Hilty, C., Wider, G. & Wuthrich, K. J. Magn. Reson. 178, 72–76 (2006).
Gagné, S.M. et al. Protein Sci. 3, 1961–1974 (1994).
Wang, Y., Zhao, S., Somerville, R.L. & Jardetzky, O. Protein Sci. 10, 592–598 (2001).
Rückert, M. & Otting, G. J. Am. Chem. Soc. 122, 7793–7797 (2000).
Schwieters, C.D., Kuszewski, J.J., Tjandra, N. & Clore, G.M. J. Magn. Reson. 160, 65–73 (2003).
Alberts, I.L., Nadassy, K. & Wodak, S.J. Protein Sci. 7, 1700–1716 (1998).
Viles, J.H. et al. J. Mol. Biol. 279, 973–986 (1998).
Ohlenschläger, O. et al. Oncogene 25, 5953–5959 (2006).
Banci, L., Bertini, I., Del Conte, R., Mangani, S. & Meyer-Klaucke, W. Biochemistry 42, 2467–2474 (2003).
Tenderholt, A. Pyspline (Stanford University, Stanford, 2007).
Mustre de Leon, J., Rehr, J.J., Zabinsky, S.I. & Albers, R.C. Phys. Rev. B. Condens. Matter 44, 4146–4156 (1991).
Rehr, J.J. & Albers, R.C. Rev. Mod. Phys. 72, 621–654 (2000).
Rehr, J.J., Deleon, J.M., Zabinsky, S.I. & Albers, R.C. J. Am. Chem. Soc. 113, 5135–5140 (1991).
Kim, C.A. & Berg, J.M. Nat. Struct. Biol. 3, 940–945 (1996).
George, G.N. EXAFSPAK and EDG-FIT (Stanford Synchrotron Radiation Lightsource, Menlo Park, 2000).
Acknowledgements
We thank M. Golynskiy and A. Pohorille for helpful discussions; Z. Sachs, F.P. Seebeck, J.W. Szostak and F. Hollfelder for comments on the manuscript; and R. Majerle for isothermal titration calorimetry instrument use. This work was supported by the US National Aeronautics and Space Administration (NASA) Agreement no. NNX09AH70A through the NASA Astrobiology Institute–Ames Research Center (to F.-A.C., A.M., L.C. and B.S.); the Minnesota Medical Foundation (to B.S.) and the US National Institutes of Health (NIH) (T32 GM08347 to J.C.H., T32 DE007288 to L.R.M., GM100310 to G.V. and P41 RR001209). Stanford Synchrotron Radiation Lightsource (SSRL) operations are funded by the US Department of Energy (DOE)–Basic Energy Sciences. The SSRL Structural Molecular Biology program is supported by NIH–National Center for Research Resources and DOE–Biological Environmental Resarch.
Author information
Authors and Affiliations
Contributions
G.V. and B.S. designed the project; A.M., J.C.H., L.C. and L.N.H. expressed and assayed proteins; F.-A.C. carried out all NMR and isothermal titration calorimetry experiments; F.-A.C. and L.S. calculated the structure; R.S. performed the EXAFS measurements, all authors analyzed the data; and F.-A.C., L.R.M., G.V. and B.S. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results (PDF 1859 kb)
Rights and permissions
About this article
Cite this article
Chao, FA., Morelli, A., III, J. et al. Structure and dynamics of a primordial catalytic fold generated by in vitro evolution. Nat Chem Biol 9, 81–83 (2013). https://doi.org/10.1038/nchembio.1138
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1138
This article is cited by
-
Experimental characterization of de novo proteins and their unevolved random-sequence counterparts
Nature Ecology & Evolution (2023)
-
d-Amino acid substituted peptides as potential alternatives of homochiral l-configurations
Amino Acids (2021)
-
Rescue of conformational dynamics in enzyme catalysis by directed evolution
Nature Communications (2018)
-
Comparing proteins and nucleic acids for next-generation biomolecular engineering
Nature Reviews Chemistry (2018)
-
The relationship between folding and activity in UreG, an intrinsically disordered enzyme
Scientific Reports (2017)