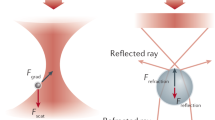
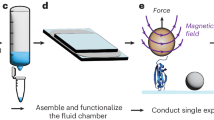
Abstract
Biological systems can be quantitatively explored using single-molecule manipulation techniques such as optical or magnetic tweezers or atomic force microscopy. Though a plethora of discoveries have been accomplished using single-molecule manipulation techniques in vitro, such investigations constantly face the criticism that conditions are too far from being physiologically relevant. Technical achievements now allow scientists to take the next step: to use single-molecule manipulation techniques quantitatively in vivo. Considerable progress has been accomplished in this realm; for example, the interaction between a protein and the membrane of a living cell has been probed, the mechanical properties of individual proteins central for cellular adhesion have been measured and even the action of molecular motors in living cells has been quantified. Here, we review the progress of in vivo single-molecule manipulation with a focus on the special challenges posed by in vivo conditions and how these can be overcome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Block, S.M., Goldstein, L.S. & Schnapp, B.J. Bead movement by single kinesin molecules studied with optical tweezers. Nature 348, 348–352 (1990).
Schnitzer, M.J. & Block, S.M. Kinesin hydrolyses one ATP per 8-nm step. Nature 388, 386–390 (1997).
Shaevitz, J.W., Abbondanzieri, E.A., Landick, R. & Block, S.M. Backtracking by single RNA polymerase molecules observed at near-base-pair resolution. Nature 426, 684–687 (2003).
Wang, M.D. Force and velocity measured for single molecules of RNA polymerase. Science 282, 902–907 (1998).
Strick, T.R., Croquette, V. & Bensimon, D. Single-molecule analysis of DNA uncoiling by a type II topoisomerase. Nature 404, 901–904 (2000).
Wen, J.D. et al. Following translation by single ribosomes one codon at a time. Nature 452, 598–603 (2008).
Charvin, G., Strick, T.R., Bensimon, D. & Croquette, V. Tracking topoisomerase activity at the single-molecule level. Annu. Rev. Biophys. Biomol. Struct. 34, 201–219 (2005).
Koster, D.A., Palle, K., Bot, E.S., Bjornsti, M.A. & Dekker, N.H. Antitumour drugs impede DNA uncoiling by topoisomerase I. Nature 448, 213–217 (2007).
Wang, M.D., Yin, H., Landick, R., Gelles, J. & Block, S. Stretching DNA with optical tweezers. Biophys. J. 72, 1335–1346 (1997).
van Mameren, J. et al. Unraveling the structure of DNA during overstretching by using multicolor, single-molecule fluorescence imaging. Proc. Natl. Acad. Sci. USA 106, 18231–18236 (2009).
Gross, P. et al. Quantifying how DNA stretches, melts and changes twist under tension. Nat. Phys. 7, 731–736 (2011).
Rief, M., Gautel, M., Oesterhelt, F., Fernandez, J.M. & Gaub, H.E. Reversible unfolding of individual titin immunoglobulin domains by AFM. Science 276, 1109–1112 (1997).
Fernandez, J.M. & Li, H.B. Force-clamp spectroscopy monitors the folding trajectory of a single protein. Science 303, 1674–1678 (2004).
Winther, T., Xu, L., Berg-Sørensen, K., Brown, S. & Oddershede, L. Effect of energy metabolism on protein motility in bacterial outer membranes. Biophys. J. 97, 1305–1312 (2009). First proof that in vivo membrane protein motility is dependent on the cell's physiological state.
Gross, S.P., Welte, M.A., Block, S.M. & Wieschaus, E.F. Dynein-mediated cargo transport in vivo. A switch controls travel distance. J. Cell Biol. 148, 945–956 (2000).
Wang, Z., Khan, S. & Sheetz, M.P. Single cytoplasmic dynein molecule movements: characterization and comparison with kinesin. Biophys. J. 69, 2011–2023 (1995).
Sørensen, M.A. & Pedersen, S. Absolute in vivo translation rates of individual codons in Escherichia coli. The two glutamic acid codons GAA and GAG are translated with a threefold difference in rate. J. Mol. Biol. 222, 265–280 (1991).
Binnig, G., Quate, C.F. & Gerber, C. Atomic force microscope. Phys. Rev. Lett. 56, 930–933 (1986).
Ashkin, A. & Dziedzic, J. Optical trapping and manipulation of viruses and bacteria. Science 235, 1517–1520 (1987). This paper showed that microorganisms could stay alive while optically trapped, thus paving the way for in vivo single-molecule investigations.
Ashkin, A., Schutze, K., Dziedzic, J., Euteneuer, U. & Schliwa, M. Force generation of organelle transport measured in vivo by an infrared laser trap. Nature 348, 346–348 (1990). First quantitative in vivo measurements of the force exerted by an individual molecular motor.
Amblard, F., Yurke, B., Pargellis, A. & Leibler, S. A magnetic manipulator for studying local rheology and micromechanical properties of biological systems. Rev. Sci. Instrum. 67, 818–827 (1996).
Gosse, C. & Croquette, V. Magnetic tweezers: micromanipulation and force measurement at the molecular level. Biophys. J. 82, 3314–3329 (2002).
de Vries, A.H., Krenn, B.E., van Driel, R. & Kanger, J.S. Micro magnetic tweezers for nanomanipulation inside live cells. Biophys. J. 88, 2137–2144 (2005).
Evans, E., Ritchie, K. & Merkel, R. Sensitive force technique to probe molecular adhesion and structural linkages at biological interfaces. Biophys. J. 68, 2580–2587 (1995).
Neuman, K.C. & Nagy, A. Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. Nat. Methods 5, 491–505 (2008). A comprehensive review of the methods central for single molecule manipulation and of the status of the in vitro field.
Moffitt, J.R., Chemla, Y., Smith, S. & Bustamante, C. Recent advances in optical tweezers. Annu. Rev. Biochem. 77, 205–228 (2008).
Stevenson, D.J., Gunn-Moore, F. & Dholakia, K. Light forces the pace: optical manipulation for biophotonics. J. Biomed. Opt. 15, 041503 (2010).
Veigel, C. & Schmidt, C.F. Moving into the cell: single-molecule studies of molecular motors in complex environments. Nat. Rev. Mol. Cell Biol. 12, 163–176 (2011).
Dufrêne, Y.F. et al. Five challenges to bringing single-molecule force spectroscopy into living cells. Nat. Methods 8, 123–127 (2011).
Valle, F. et al. A polymeric molecular “handle” for multiple AFM-based single-molecule force measurements. Angew. Chem. Int. Ed. Engl. 47, 2431–2434 (2008).
Jauffred, L., Richardson, A. & Oddershede, L. Three-dimensional optical control of individual quantum dots. Nano Lett. 8, 3376–3380 (2008).
Selhuber-Unkel, C., Zins, I., Schubert, O., Soennichsen, C. & Oddershede, L. Quantitative optical trapping of single gold nanorods. Nano Lett. 8, 2998–3003 (2008).
Ma, H., Bendix, P. & Oddershede, L. Large-scale orientation dependent heating from a single irradiated gold nanorod. Nano Lett. 12, 3954–3960 (2012).
Merkel, R. & Nassoy, P. Leung, A., Ritchie, K. & Evans, E. Energy landscapes of receptor-ligand bonds explored with dynamic force spectroscopy. Nature 397, 50–53 (1999).
Evans, E. & Ritchie, K. Dynamic strength of molecular adhesion bonds. Biophys. J. 72, 1541–1555 (1997).
Evans, E.A. & Calderwood, D. Forces and bond dynamics in cell adhesion. Science 316, 1148–1153 (2007).
Oddershede, L., Dreyer, J.K., Grego, S., Brown, S. & Berg-Sørensen, K. The motion of a single molecule, the l-receptor, in the bacterial outer membrane. Biophys. J. 83, 3152–3161 (2002).
Huang, J., Nagy, S.S., Koide, A., Rock, R.S. & Koide, S. A peptide tag system for facile purification and single-molecule immobilization. Biochemistry 48, 11834–11836 (2009).
Wang, S.H., Lee, C.W., Chiou, A. & Wei, P.K. Size-dependent endocytosis of gold nanoparticles studied by three-dimensional mapping of plasmonic scattering images. J. Nanobiotechnology 8, 33 (2010).
Iversen, T.G., Skotland, T. & Sandvig, K. Endocytosis and intracellular transport of nanoparticles: present knowledge and need for future studies. Nano Today 6, 176–185 (2011).
Marchington, R.F., Arita, Y., Tsampoula, X., Gunn-Moore, F.J. & Dholakia, K. Optical injection of mammalian cells using a microfluidic platform. Biomed. Opt. Express 1, 527–536 (2010).
Nicklas, R.B. & Koch, C.A. Chromosome micromanipulation. 3. Spindle fiber tension and the reorientation of mal-oriented chromosomes. J. Cell Biol. 43, 40–50 (1969).
Edidin, M., Kuo, S.C. & Sheetz, M.P. Lateral movements of membrane glycoproteins restricted by dynamic cytoplasmic barriers. Science 254, 1379–1382 (1991).
Sako, Y. & Kusumi, A. Barriers for lateral diffusion of transferrin receptor in the plasma membrane as characterized by receptor dragging by laser tweezers: fence versus tether. J. Cell Biol. 129, 1559–1574 (1995). One of the first papers to show how individual membrane proteins can be individually manipulated in vivo.
Tomishige, M. & Kusumi, A. Compartmentalization of the erythrocyte membrane by the membrane skeleton: intercompartmental hop diffusion of band 3. Mol. Biol. Cell 10, 2475–2479 (1999).
Pralle, A., Keller, P., Florin, E.L., Simons, K. & Hörber, J.K. Sphingolipidcholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J. Cell Biol. 148, 997–1008 (2000). Demonstration of an elegant method to probe the interaction between a single protein and the membrane in the protein's local environment.
Suzuki, K., Ritchie, K., Kajikawa, E., Fujiwara, T. & Kusumi, A. Rapid hop diffusion of a G-protein–coupled receptor in the plasma membrane as revealed by single-molecule techniques. Biophys. J. 88, 3659–3680 (2005).
Gibbs, K.A. et al. Complex spatial distribution and dynamics of an abundant Escherichia coli outer membrane protein, LamB. Mol. Microbiol. 53, 1771–1783 (2004).
Winther, T. & Oddershede, L.B. Effect of antibiotics and antimicrobial peptides on single protein motility. Curr. Pharm. Biotechnol. 10, 486–493 (2009).
Rieger, B., Dietrich, H., van den Doel, L. & van Vliet, L. Diffusion of micro-spheres in sealed and open microarrays. Microsc. Res. Tech. 65, 218–225 (2004).
Benoit, M., Gabriel, D., Gerisch, G. & Gaub, H.E. Discrete interactions in cell adhesion measured by single-molecule force spectroscopy. Nat. Cell Biol. 2, 313–317 (2000). Among the first reports of high-quality AFM measurements of adhesion forces at the single-molecule level.
Benoit, M. & Gaub, H.E. Measuring cell adhesion forces with the atomic force microscope at the molecular level. Cells Tissues Organs 172, 174–189 (2002).
Franz, C.M., Taubenberger, A., Puech, P.H. & Muller, D.J. Studying integrin-mediated cell adhesion at the single-molecule level using AFM force spectroscopy. Sci. STKE 2007, pl5 (2007).
Heinisch, J., Dupres, V., Wilk, S., Jendretzki, A. & Dufrene, Y. Single-molecule atomic force microscopy reveals clustering of the yeast plasma-membrane sensor Wsc1. PLoS ONE 5, e11104 (2010).
Grashoff, C. et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466, 263–266 (2010).
Welte, M.A., Gross, S.P., Postner, M., Block, S.M. & Wieschaus, E.F. Developmental regulation of vesicle transport in Drosophila embryos: forces and kinetics. Cell 92, 547–557 (1998).
Sims, P.A. & Xie, X.S. Probing dynein and kinesin stepping with mechanical manipulation in a living cell. Chemphyschem. 10, 1511–1516 (2009). In vivo quantitative measurements of stepping sizes, stall forces and cooperativity of dynein and kinesin inside living cells.
Shubeita, G.T. et al. Consequences of motor copy number on the intracellular transport of kinesin-1–driven lipid droplets. Cell 135, 1098–1107 (2008).
Shtridelman, Y., Cahyuti, T., Townsend, B., DeWitt, D. & Macosko, J.C. Force-velocity curves of motor proteins cooperating in vivo. Cell Biochem. Biophys. 52, 19–29 (2008).
Xu, J. et al. Casein kinase 2 reverses tail-independent inactivation of kinesin-1. Nat. Commun. 3, 754 (2012).
Iwai, S. & Uyeda, T. Visualizing myosin-actin interaction with a genetically-encoded fluorescent strain sensor. Proc. Natl. Acad. Sci. USA 105, 16882–16887 (2008).
Pierobon, P. et al. Velocity, processivity, and individual steps of single myosin V molecules in live cells. Biophys. J. 96, 4268–4275 (2009). Study of differences and similarities between myosin V motility in vitro and in vivo.
Robert, D., Nguyen, T.H., Gallet, F. & Wilhelm, C. In vivo determination of fluctuating forces during endosome trafficking using a combination of active and passive microrheology. PLoS ONE 5, e10046 (2010).
Sun, M., Kawamura, R. & Marko, J.F. Micromechanics of human mitotic chromosomes. Phys. Biol. 8, 015003 (2011).
Shimamoto, Y., Maeda, Y.T., Ishiwata, S., Libchaber, A.J. & Kapoor, T.M. Insights into the micromechanical properties of the metaphase spindle. Cell 145, 1062–1074 (2011).
Laan, L., Husson, J., Munteanu, E.L., Kerssemakers, J.W.J. & Dogterom, M. Force-generation and dynamic instability of microtubule bundles. Proc. Natl. Acad. Sci. USA 105, 8920–8925 (2008).
Footer, M.J., Kerssemakers, J.W.J., Theriot, J.A. & Dogterom, M. Direct measurement of force generation by actin filament polymerization using an optical trap. Proc. Natl. Acad. Sci. USA 104, 2181–2186 (2007).
Hsiao, S.C. et al. DNA-coated AFM cantilevers for the investigation of cell adhesion and the patterning of live cells. Angew. Chem. Int. Ed. Engl. 47, 8473–8477 (2008).
Lang, M.J., Fordyce, P.M., Engh, A.M., Neuman, K.C. & Block, S.M. Simultaneous, coincident optical trapping and single-molecule fluorescence. Nat. Methods 1, 133–139 (2004).
Lee, W.M., Reece, P.J., Marchington, R.F., Metzger, N.K. & Dholakia, K. Construction and calibration of an optical trap on a fluorescence optical microscope. Nat. Protoc. 2, 3226–3238 (2007).
Vickery, S.A. & Dunn, R.C. Combining AFM and FRET for high resolution fluorescence microscopy. J. Microsc. 202, 408–412 (2001).
Hernando, J. et al. Investigation of perylene photonic wires by combined single-molecule fluorescence and atomic force microscopy. Angew. Chem. Int. Ed. Engl. 43, 4045–4049 (2004).
Rust, M.J., Bates, M. & Zhuang, X. Imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 3, 793–795 (2006).
Fölling, J. et al. Fluorescence nanoscopy by ground-state depletion and single-molecule return. Nat. Methods 5, 943–945 (2008).
Hell, S.W. Microscopy and its focal switch. Nat. Methods 6, 24–32 (2009).
Finkelstein, I.J., Visnapuu, M.-L. & Greene, E.C. Single-molecule imaging reveals mechanisms of protein disruption by a DNA translocase. Nature 468, 983–987 (2010).
Jin, J. et al. Synergistic action of RNA polymerases in overcoming the nucleosomal barrier. Nat. Struct. Mol. Biol. 17, 745–752 (2010).
Curtis, J., Koss, B. & Grier, D. Dynamic holographic optical tweezers. Opt. Commun. 207, 169–175 (2002).
Garcés-Chávez, V., McGloin, D., Melville, H., Sibbett, W. & Dholakia, K. Simultaneous micromanipulation in multiple planes using a self-reconstructing light beam. Nature 419, 145–147 (2002).
Tolić-Nørrelykke, I.M., Munteanu, E.L., Thon, G., Oddershede, L. & Berg-Sørensen, K. Anomalous diffusion in living yeast cells. Phys. Rev. Lett. 93, 078102 (2004).
Jeon, J.H. et al. In vivo anomalous diffusion and weak ergodicity breaking of lipid granules. Phys. Rev. Lett. 106, 048103 (2011).
Svoboda, K. & Block, S.M. Optical trapping of metallic Rayleigh particles. Opt. Lett. 19, 930–932 (1994).
Hansen, P.M., Bhatia, V., Harrit, N. & Oddershede, L. Expanding the optical trapping range of gold nanoparticles. Nano Lett. 5, 1937–1942 (2005).
Seol, Y., Carpenter, A.E. & Perkins, T.T. Gold nanoparticles: enhanced optical trapping and sensitivity coupled with significant heating. Opt. Lett. 31, 2429–2431 (2006).
Liang, H. et al. Wavelength dependence of cell cloning efficiency after optical trapping. Biophys. J. 70, 1529–1533 (1996).
Leitz, G., Fällman, E., Tuck, S. & Axner, O. Stress response in Caenorhabditis elegans caused by optical tweezers: wavelength, power, and time dependence. Biophys. J. 82, 2224–2231 (2002).
Neuman, K.C., Chadd, E., Liou, G., Bergman, K. & Block, S. Characterization of photodamage to Escherichia coli in optical traps. Biophys. J. 77, 2856–2863 (1999). Among the first quantifications of physiological damage exerted by optical trapping of living organisms.
Rasmussen, M.B., Oddershede, L. & Siegumfeldt, H. Optical tweezers cause physiological damage to E. coli and Listeria bacteria. Appl. Environ. Microbiol. 74, 2441–2446 (2008).
Peterman, E.J., Gittes, F. & Schmidt, C. Laser-induced heating in optical traps. Biophys. J. 84, 1308–1316 (2003).
Bendix, P.M., Reihani, S.N.S. & Oddershede, L.B. Direct measurements of heating by electromagnetically trapped gold nanoparticles on supported lipid bilayers. ACS Nano 4, 2256–2262 (2010).
Berg-Sørensen, K. & Flyvbjerg, H. Power spectrum analysis for optical tweezers. Rev. Sci. Instrum. 75, 594–612 (2004).
Gittes, F. & Schmidt, C.F. Interference model for back-focal-plane displacement detection in optical tweezers. Opt. Lett. 23, 7–9 (1998).
Tolić-Nørrelykke, S.F., Schaffer, E., Howard, J., Pavone, F.S., Julicher, F. & Flyvbjerg, H. Calibration of optical tweezers with positional detection in the back focal plane. Rev. Sci. Instrum. 77, 103101 (2006).
Fischer, M. & Berg-Sørensen, K. Calibration of trapping force and response function of optical tweezers in viscoelastic media. J. Opt. A: Pure Appl. Opt. 9, S239–S250 (2007).
Fischer, M., Richardson, A.C., Reihani, S.N.S., Oddershede, L.B. & Berg-Sørensen, K. Active-passive calibration of optical tweezers in viscoelastic media. Rev. Sci. Instrum. 81, 015103 (2010).
Zlatanova, J. & Leuba, S. Magnetic tweezers: a sensitive tool to study DNA and chromatin at the single molecule level. Biochem. Cell Biol. 81, 151–159 (2003).
Lipfert, J., Kerssemakers, J., Jager, T. & Dekker, N. Magnetic torque tweezers: measuring torsional stiffness in DNA and RecA-DNA filaments. Nat. Methods 7, 977–980 (2010).
La Porta, A. & Wang, M.D. Optical torque wrench: angular trapping, rotation and torque detection of quartz microspheres. Phys. Rev. Lett. 92, 190801 (2004).
Müller, D.J., Helenius, J., Alsteens, D. & Dufrêne, Y.F. Force probing surfaces of living cells to molecular resolution. Nat. Chem. Biol. 5, 383–390 (2009). Recommendable review of how AFMs have been used to probe properties of living cells' surfaces at the single-molecule level.
Thomas, W.E., Vogel, V. & Sokurenko, E. Biophysics of catch bonds. Annu. Rev. Biophys. 37, 399–416 (2008).
Acknowledgements
The author acknowledges useful comments from E.J.G. Peterman, N.H. Dekker, C. Selhuber-Unkel and M.A. Sørensen and assistance with the illustrations from artist M. Høst. The author acknowledges financial support from the University of Copenhagen Excellence Program and from the Lundbeck Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Oddershede, L. Force probing of individual molecules inside the living cell is now a reality. Nat Chem Biol 8, 879–886 (2012). https://doi.org/10.1038/nchembio.1082
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1082
This article is cited by
-
Direct manipulation of liquid ordered lipid membrane domains using optical traps
Communications Chemistry (2019)
-
Optical manipulation from the microscale to the nanoscale: fundamentals, advances and prospects
Light: Science & Applications (2017)
-
Optical micromanipulation of nanoparticles and cells inside living zebrafish
Nature Communications (2016)
-
Applications of Atomic Force Microscopy in Exploring Drug Actions in Lymphoma-Targeted Therapy at the Nanoscale
BioNanoScience (2016)
-
Nanoscale monitoring of drug actions on cell membrane using atomic force microscopy
Acta Pharmacologica Sinica (2015)