Abstract
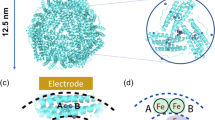
A conserved iron-binding site, the ferroxidase center, regulates the vital iron storage role of the ubiquitous protein ferritin in iron metabolism. It is commonly thought that two Fe(II) simultaneously bind the ferroxidase center and that the oxidized Fe(III)-O(H)-Fe(III) product spontaneously enters the cavity of ferritin as a unit. In contrast, in some bacterioferritins and in archaeal ferritins a persistent di-iron prosthetic group in this center is believed to mediate catalysis of core formation. Using a combination of binding experiments and isotopically labeled 57Fe(II), we studied two systems in comparison: the ferritin from the hyperthermophilic archaeal anaerobe Pyrococcus furiosus (PfFtn) and the eukaryotic human H ferritin (HuHF). The results do not support either of the two paradigmatic models; instead they suggest a unifying mechanism in which the Fe(III)-O-Fe(III) unit resides in the ferroxidase center until it is sequentially displaced by Fe(II).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
19 April 2013
In the version of this article initially published, the units (kJ mol−1) associated with the ΔH values reported in Table 1 were inadvertently omitted. The error has been corrected in the HTML and PDF versions of the article.
References
Theil, E.C. Ferritin—structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu. Rev. Biochem. 56, 289–315 (1987).
Treffry, A., Harrison, P.M., Cleton, M.I., de Bruijn, W.C. & Mann, S. A note on the composition and properties of ferritin iron cores. J. Inorg. Biochem. 31, 1–6 (1987).
Tatur, J., Hagedoorn, P.-L., Overeijnder, M. & Hagen, W. A highly thermostable ferritin from the hyperthermophilic archaeal anaerobe Pyrococcus furiosus. Extremophiles 10, 139–148 (2006).
Rucker, P., Torti, F.M. & Torti, S.V. Role of H and L subunits in mouse ferritin. J. Biol. Chem. 271, 33352–33357 (1996).
Dickey, L.F. et al. Differences in the regulation of messenger RNA for housekeeping and specialized-cell ferritin. A comparison of three distinct ferritin complementary DNAs, the corresponding subunits, and identification of the first processed in amphibia. J. Biol. Chem. 262, 7901–7907 (1987).
Cheesman, M.R., Thomson, A.J., Greenwood, C., Moore, G.R. & Kadir, F. Bis-methionine axial ligation of haem in bacterioferritin from Pseudomonas aeruginosa. Nature 346, 771–773 (1990).
Arosio, P. & Levi, S. Ferritin, iron homeostasis, and oxidative damage. Free Radic. Biol. Med. 33, 457–463 (2002).
Ferreira, C. et al. Early embryonic lethality of H ferritin gene deletion in mice. J. Biol. Chem. 275, 3021–3024 (2000).
Lange, S.J. & Que, L. Oxygen activating nonheme iron enzymes. Curr. Opin. Chem. Biol. 2, 159–172 (1998).
Tatur, J., Hagen, W. & Matias, P. Crystal structure of the ferritin from the hyperthermophilic archaeal anaerobe Pyrococcus furiosus. J. Biol. Inorg. Chem. 12, 615–630 (2007).
Stillman, T.J. et al. The high-resolution X-ray crystallographic structure of the ferritin (EcFtnA) of Escherichia coli; comparison with human H ferritin (HuHF) and the structures of the Fe3+ and Zn2+ derivatives. J. Mol. Biol. 307, 587–603 (2001).
Yao, H. et al. Two distinct ferritin-like molecules in Pseudomonas aeruginosa: the product of the bfrA gene is a bacterial ferritin (FtnA) and not a bacterioferritin (Bfr). Biochemistry 50, 5236–5248 (2011).
Treffry, A., Hirzmann, J., Yewdall, S.J. & Harrison, P.M. Mechanism of catalysis of Fe(II) oxidation by ferritin H chains. FEBS Lett. 302, 108–112 (1992).
Le Brun, N.E., Crow, A., Murphy, M.E.P., Mauk, A.G. & Moore, G.R. Iron core mineralisation in prokaryotic ferritins. Biochim. Biophys. Acta 1800, 732–744 (2010).
Hwang, J. et al. A short Fe-Fe distance in peroxodiferric ferritin: control of Fe substrate versus cofactor decay? Science 287, 122–125 (2000).
Treffry, A., Zhao, Z., Quail, M.A., Guest, J.R. & Harrison, P.M. How the presence of three iron binding sites affects the iron storage function of the ferritin (EcFtnA) of Escherichia coli. FEBS Lett. 432, 213–218 (1998).
Tatur, J. & Hagen, W.R. The dinuclear iron-oxo ferroxidase center of Pyrococcus furiosus ferritin is a stable prosthetic group with unexpectedly high reduction potentials. FEBS Lett. 579, 4729–4732 (2005).
Honarmand Ebrahimi, K., Hagedoorn, P.-L., Jongejan, J. & Hagen, W. Catalysis of iron core formation in Pyrococcus furiosus ferritin. J. Biol. Inorg. Chem. 14, 1265–1274 (2009).
Weeratunga, S.K. et al. Structural studies of bacterioferritin B from Pseudomonas aeruginosa suggest a gating mechanism for iron uptake via the ferroxidase center. Biochemistry 49, 1160–1175 (2010).
Crow, A., Lawson, T.L., Lewin, A., Moore, G.R. & Brun, N.E.L. Structural basis for iron mineralization by bacterioferritin. J. Am. Chem. Soc. 131, 6808–6813 (2009).
Leavitt, S. & Freire, E. Direct measurement of protein binding energetics by isothermal titration calorimetry. Curr. Opin. Struct. Biol. 11, 560–566 (2001).
Levi, S. et al. Expression and structural and functional properties of human ferritin L-chain from Escherichia coli. Biochemistry 28, 5179–5184 (1989).
Levi, S. et al. Mechanism of ferritin iron uptake: activity of the H-chain and deletion mapping of the ferro-oxidase site. A study of iron uptake and ferro-oxidase activity of human liver, recombinant H-chain ferritins, and of two H-chain deletion mutants. J. Biol. Chem. 263, 18086–18092 (1988).
Lawson, D.M. et al. Solving the structure of human H ferritin by genetically engineering intermolecular crystal contacts. Nature 349, 541–544 (1991).
Masuda, T., Goto, F., Yoshihara, T. & Mikami, B. The universal mechanism for iron translocation to the ferroxidase site in ferritin, which is mediated by the well conserved transit site. Biochem. Biophys. Res. Commun. 400, 94–99 (2010).
Masuda, T., Goto, F., Yoshihara, T. & Mikami, B. Crystal structure of plant ferritin reveals a novel metal binding site that functions as a transit site for metal transfer in ferritin. J. Biol. Chem. 285, 4049–4059 (2010).
Andrews, S.C. The Ferritin-like superfamily: evolution of the biological iron storeman from a rubrerythrin-like ancestor. Biochim. Biophys. Acta 1800, 691–705 (2010).
Mathevon, C. et al. tRNA-modifying MiaE protein from Salmonella typhimurium is a nonheme diiron monooxygenase. Proc. Natl. Acad. Sci. USA 104, 13295–13300 (2007).
Bou-Abdallah, F., Zhao, G., Mayne, H.R., Arosio, P. & Chasteen, N.D. Origin of the unusual kinetics of iron deposition in human H-chain ferritin. J. Am. Chem. Soc. 127, 3885–3893 (2005).
Pereira, A.S. et al. Direct spectroscopic and kinetic evidence for the involvement of a peroxodiferric intermediate during the ferroxidase reaction in fast ferritin mineralization. Biochemistry 37, 9871–9876 (1998).
St. Pierre, T.G. et al. Mössbauer spectroscopic studies of the cores of human, limpet and bacterial ferritins. Biochim. Biophys. Acta 870, 127–134 (1986).
Mann, S., Williams, J.M., Treffry, A. & Harrison, P.M. Reconstituted and native iron-cores of bacterioferritin and ferritin. J. Mol. Biol. 198, 405–416 (1987).
Paulsen, K.E. et al. Oxidation-reduction potentials of the methane monooxygenase hydroxylase component from Methylosinus trichosporium OB3b. Biochemistry 33, 713–722 (1994).
Chasteen, N.D., Antanaitis, B.C. & Aisen, P. Iron deposition in apoferritin. Evidence for the formation of a mixed valence binuclear iron complex. J. Biol. Chem. 260, 2926–2929 (1985).
Bauminger, E.R. et al. Iron (II) oxidation and early intermediates of iron-core formation in recombinant human H-chain ferritin. Biochem. J. 296, 709–719 (1993).
Bou-Abdallah, F. et al. μ-1,2-Peroxobridged diiron(III) dimer formation in human H-chain ferritin. Biochem. J. 364, 57–63 (2002).
Bauminger, E.R. et al. Stages in iron storage in the ferritin of Escherichia coli (EcFtnA):analysis of Mössbauer spectra reveals a new intermediate. Biochemistry 38, 7791–7802 (1999).
Turano, P., Lalli, D., Felli, I.C., Theil, E.C. & Bertini, I. NMR reveals pathway for ferric mineral precursors to the central cavity of ferritin. Proc. Natl. Acad. Sci. USA 107, 545–550 (2010).
Ha, Y., Shi, D., Small, G.W., Theil, E.C. & Allewell, N.M. Crystal structure of bullfrog M ferritin at 2.8-Å resolution: analysis of subunit interactions and the binuclear metal center. J. Biol. Inorg. Chem. 4, 243–256 (1999).
Tosha, T., Ng, H.-L., Bhattasali, O., Alber, T. & Theil, E.C. Moving metal ions through ferritin−protein nanocages from three-fold pores to catalytic sites. J. Am. Chem. Soc. 132, 14562–14569 (2010).
Bertini, I. et al. Structural insights into the ferroxidase site of ferritins from higher eukaryotes. J. Am. Chem. Soc. 134, 6169–6176 (2012).
Hagen, W.R. Biomolecular EPR Spectroscopy (CRC Press, 2009).
Hagen, W.R. EPR spectroscopy as a probe of metal centres in biological systems. Dalton Trans. 4415–4434 (2006).
Acknowledgements
The construct for production of recombinant HuHF was kindly provided by P. Arosio (University of Brescia). We thank L. van der Weel for assistance with expression of ferritin, M.J.F. Strampraad for technical assistance, D. Sordi for her assistance with the E129R mutation, M.M. Kabir for assistance with kinetic measurements and B. Mienert for Mössbauer measurements. This work was financially supported by a research grant from the Dutch National Research School Combination–Catalysis Controlled by Chemical Design (NRSC-C).
Author information
Authors and Affiliations
Contributions
K.H.E., P.-L.H. and W.R.H. designed the research; K.H.E. performed the experiments; K.H.E., P.-L.H., E.B. and W.R.H. analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Results (PDF 2686 kb)
Rights and permissions
About this article
Cite this article
Honarmand Ebrahimi, K., Bill, E., Hagedoorn, PL. et al. The catalytic center of ferritin regulates iron storage via Fe(II)-Fe(III) displacement. Nat Chem Biol 8, 941–948 (2012). https://doi.org/10.1038/nchembio.1071
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1071
This article is cited by
-
A core-satellite-like nanoassembly reverses a decisive tyrosine hydroxylase loss in degenerative dopaminergic neurons
Nano Research (2023)
-
Identification of novel yolk ferritins unique to planarians: planarians supply aluminum rather than iron to vitellaria in egg capsules
Cell and Tissue Research (2021)
-
Iron redox pathway revealed in ferritin via electron transfer analysis
Scientific Reports (2020)
-
Biogenic manganese oxide nanoparticle formation by a multimeric multicopper oxidase Mnx
Nature Communications (2017)
-
Accurate label-free reaction kinetics determination using initial rate heat measurements
Scientific Reports (2015)